Effective Protection Induced by a Monovalent DNA Vaccine against Dengue Virus (DV) Serotype 1 and a Bivalent DNA Vaccine against DV1 and DV2 in Mice
- PMID: 28553618
- PMCID: PMC5427067
- DOI: 10.3389/fcimb.2017.00175
Effective Protection Induced by a Monovalent DNA Vaccine against Dengue Virus (DV) Serotype 1 and a Bivalent DNA Vaccine against DV1 and DV2 in Mice
Abstract
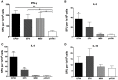
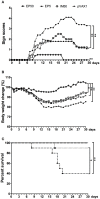
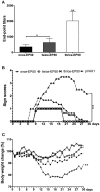
Dengue virus (DV) is the causal pathogen of dengue fever, which is one of the most rapidly spread mosquito-borne disease worldwide and has become a severe public health problem. Currently, there is no specific treatment for dengue; thus, a vaccine would be an effective countermeasure to reduce the morbidity and mortality. Although, the chimeric Yellow fever dengue tetravalent vaccine has been approved in some countries, it is still necessary to develop safer, more effective, and less costly vaccines. In this study, a DNA vaccine candidate pVAX1-D1ME expressing the prME protein of DV1 was inoculated in BALB/c mice via intramuscular injection or electroporation, and the immunogenicity and protection were evaluated. Compared with traditional intramuscular injection, administration with 50 μg pVAX1-D1ME via electroporation with three immunizations induced persistent humoral and cellular immune responses and effectively protected mice against lethal DV1 challenge. In addition, immunization with a bivalent vaccine consisting of pVAX1-D1ME and pVAX1-D2ME via electroporation generated a balanced IgG response and neutralizing antibodies against DV1 and DV2 and could protect mice from lethal challenge with DV1 and DV2. This study sheds new light on developing a dengue tetravalent DNA vaccine.
Keywords: DNA vaccine; bivalent vaccine; dengue; dengue virus; electroporation; monovalent vaccine.
Figures





Similar articles
-
Immunization with electroporation enhances the protective effect of a DNA vaccine candidate expressing prME antigen against dengue virus serotype 2 infection.Clin Immunol. 2016 Oct;171:41-49. doi: 10.1016/j.clim.2016.08.021. Epub 2016 Aug 28. Clin Immunol. 2016. PMID: 27578400
-
Electroporation-Mediated Immunization of a Candidate DNA Vaccine Expressing Dengue Virus Serotype 4 prM-E Antigen Confers Long-Term Protection in Mice.Virol Sin. 2019 Feb;34(1):88-96. doi: 10.1007/s12250-019-00090-8. Epub 2019 Feb 21. Virol Sin. 2019. PMID: 30790202 Free PMC article.
-
Evaluation of a DNA vaccine candidate expressing prM-E-NS1 antigens of dengue virus serotype 1 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in immunogenicity and protection.Vaccine. 2011 Jan 17;29(4):763-71. doi: 10.1016/j.vaccine.2010.11.014. Epub 2010 Nov 21. Vaccine. 2011. PMID: 21095256
-
Nucleic acid (DNA) immunization as a platform for dengue vaccine development.Vaccine. 2015 Dec 10;33(50):7135-40. doi: 10.1016/j.vaccine.2015.09.102. Epub 2015 Oct 14. Vaccine. 2015. PMID: 26458805 Review.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
Cited by
-
Development of nucleic acid-based vaccines against dengue and other mosquito-borne flaviviruses: the past, present, and future.Front Immunol. 2025 Jan 7;15:1475886. doi: 10.3389/fimmu.2024.1475886. eCollection 2024. Front Immunol. 2025. PMID: 39840044 Free PMC article. Review.
-
Long-Term Protection Elicited by a DNA Vaccine Candidate Expressing the prM-E Antigen of Dengue Virus Serotype 3 in Mice.Front Cell Infect Microbiol. 2020 Mar 17;10:87. doi: 10.3389/fcimb.2020.00087. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32257963 Free PMC article.
-
Long-term protection against dengue viruses in mice conferred by a tetravalent DNA vaccine candidate.Zool Res. 2020 Jan 18;41(1):90-93. doi: 10.24272/j.issn.2095-8137.2020.016. Zool Res. 2020. PMID: 31746566 Free PMC article.
-
Dengue Virus and Vaccines: How Can DNA Immunization Contribute to This Challenge?Front Med Technol. 2021 Apr 12;3:640964. doi: 10.3389/fmedt.2021.640964. eCollection 2021. Front Med Technol. 2021. PMID: 35047911 Free PMC article. Review.
-
Vaccination With a Single Consensus Envelope Protein Ectodomain Sequence Administered in a Heterologous Regimen Induces Tetravalent Immune Responses and Protection Against Dengue Viruses in Mice.Front Microbiol. 2019 May 10;10:1113. doi: 10.3389/fmicb.2019.01113. eCollection 2019. Front Microbiol. 2019. PMID: 31134046 Free PMC article.
References
-
- Capeding M. R., Tran N. H., Hadinegoro S. R., Ismail H. I., Chotpitayasunondh T., Chua M. N., et al. . (2014). Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384, 1358–1365. 10.1016/S0140-6736(14)61060-6 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

