Neural Stem Cell Plasticity: Advantages in Therapy for the Injured Central Nervous System
- PMID: 28553634
- PMCID: PMC5427132
- DOI: 10.3389/fcell.2017.00052
Neural Stem Cell Plasticity: Advantages in Therapy for the Injured Central Nervous System
Abstract
The physiological and pathological properties of the neural germinal stem cell niche have been well-studied in the past 30 years, mainly in animals and within given limits in humans, and knowledge is available for the cyto-architectonic structure, the cellular components, the timing of development and the energetic maintenance of the niche, as well as for the therapeutic potential and the cross talk between neural and immune cells. In recent years we have gained detailed understanding of the potentiality of neural stem cells (NSCs), although we are only beginning to understand their molecular, metabolic, and epigenetic profile in physiopathology and, further, more can be invested to measure quantitatively the activity of those cells, to model in vitro their therapeutic responses or to predict interactions in silico. Information in this direction has been put forward for other organs but is still limited in the complex and very less accessible context of the brain. A comprehensive understanding of the behavior of endogenous NSCs will help to tune or model them toward a desired response in order to treat complex neurodegenerative diseases. NSCs have the ability to modulate multiple cellular functions and exploiting their plasticity might make them into potent and versatile cellular drugs.
Keywords: aging; inflammation; metabolism; microenvironment; multiple sclerosis; neural stem cells; plasticity; stroke.
Figures
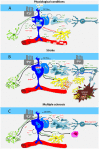
Similar articles
-
Therapeutic Plasticity of Neural Stem Cells.Front Neurol. 2020 Mar 20;11:148. doi: 10.3389/fneur.2020.00148. eCollection 2020. Front Neurol. 2020. PMID: 32265815 Free PMC article. Review.
-
Neural stem cell niches and homing: recruitment and integration into functional tissues.ILAR J. 2009;51(1):3-23. doi: 10.1093/ilar.51.1.3. ILAR J. 2009. PMID: 20075495 Review.
-
Cross-talk between stem cells and the dysfunctional brain is facilitated by manipulating the niche: evidence from an adhesion molecule.Stem Cells. 2009 Nov;27(11):2846-56. doi: 10.1002/stem.227. Stem Cells. 2009. PMID: 19785036
-
Signal transduction pathways involved in the lineage-differentiation of NSCs: can the knowledge gained from blood be used in the brain?Cancer Invest. 2004;22(6):925-43. doi: 10.1081/cnv-200039679. Cancer Invest. 2004. PMID: 15641490 Review.
-
The adult neural stem cell niche: lessons for future neural cell replacement strategies.Neurosurg Clin N Am. 2007 Jan;18(1):81-92, ix. doi: 10.1016/j.nec.2006.10.002. Neurosurg Clin N Am. 2007. PMID: 17244556 Review.
Cited by
-
Valproic Acid Labeled Chitosan Nanoparticles Promote the Proliferation and Differentiation of Neural Stem Cells After Spinal Cord Injury.Neurotox Res. 2021 Apr;39(2):456-466. doi: 10.1007/s12640-020-00304-y. Epub 2020 Nov 28. Neurotox Res. 2021. PMID: 33247828
-
Stem cell therapy for central nervous system disorders: Metabolic interactions between transplanted cells and local microenvironments.Neurobiol Dis. 2022 Oct 15;173:105842. doi: 10.1016/j.nbd.2022.105842. Epub 2022 Aug 18. Neurobiol Dis. 2022. PMID: 35988874 Free PMC article. Review.
-
Advanced hydrogel mesh platform with neural stem cells and human umbilical vein endothelial cells for enhanced axonal regeneration.APL Bioeng. 2025 Apr 1;9(2):026101. doi: 10.1063/5.0244057. eCollection 2025 Jun. APL Bioeng. 2025. PMID: 40181802 Free PMC article.
-
Reprogramming of Lipid Metabolism as a New Driving Force Behind Tauroursodeoxycholic Acid-Induced Neural Stem Cell Proliferation.Front Cell Dev Biol. 2020 May 26;8:335. doi: 10.3389/fcell.2020.00335. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582686 Free PMC article.
-
Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases.Neural Regen Res. 2022 Apr;17(4):741-747. doi: 10.4103/1673-5374.322429. Neural Regen Res. 2022. PMID: 34472459 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

