Assessment of aflatoxin B1 myocardial toxicity in rats: mitochondrial damage and cellular apoptosis in cardiomyocytes induced by aflatoxin B1
- PMID: 28553767
- PMCID: PMC5536410
- DOI: 10.1177/0300060517706579
Assessment of aflatoxin B1 myocardial toxicity in rats: mitochondrial damage and cellular apoptosis in cardiomyocytes induced by aflatoxin B1
Abstract
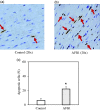
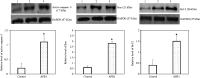
Objective The number of deaths from heart disease is increasing worldwide. Aflatoxin B1 (AFB1), a toxin produced by the fungi Aspergillus flavus and Aspergillus parasiticus, is frequently detected in improperly processed/stored human food products. While AFB1 hepatotoxicity and carcinogenic properties have been well addressed, its myocardial toxicity is poorly documented. This study aimed to investigate myocardial toxic activity of AFB1. Methods Ten rats were fed with AFB1 at a dose that did not result in acute toxic reactions for 30 days and 10 vehicle-fed rats served as controls. Transmission electron microscopy was used to assess mitochondrial damage in cardiomyocytes. The terminal deoxynucleotidyl transferase-mediated UTP nick-end labelling assay was performed to detect apoptosis of cardiomyocytes. Western blotting was performed to measure apoptotic proteins (i.e., active caspase-3, Bax, and Bcl-2) in heart tissue. Results AFB1 treatment resulted in mitochondrial membrane disruption and disorganization of cristae, which are indicators of mitochondrial damage. Myocardial cell apoptosis was significantly higher after AFB1 treatment (22.07% ± 3.29%) compared with controls (6.27% ± 2.78%, P < 0.05). AFB1 treatment enhanced expression of active caspase-3, Bax, and Bcl-2 in cardiac tissue. Conclusion Various adverse effects are exerted by AFB1 on the heart, indicating AFB1 myocardial toxicity.
Keywords: Aflatoxin B1; Bcl-2; caspase-3; cell apoptosis; mitochondria; myocardial toxicity.
Figures


References
-
- Perrone G, Gallo A. Aspergillus species and their associated mycotoxins. Methods Mol Biol 2017; 1542: 33–49. - PubMed
-
- Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol 1994; 34: 135–172. - PubMed
-
- Chen CJ, Wang LY, Lu SN, et al. Elevated aflatoxin exposure and increased risk of hepatocellular carcinoma. Hepatology 1996; 24: 38–42. - PubMed
-
- Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis 2013; 22: 305–310. - PubMed
-
- Massey TE, Smith GB, Tam AS. Mechanisms of aflatoxin B1 lung tumorigenesis. Exp Lung Res 2000; 26: 673–683. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

