Role of TAZ in Lysophosphatidic Acid-Induced Migration and Proliferation of Human Adipose-Derived Mesenchymal Stem Cells
- PMID: 28554198
- PMCID: PMC5499612
- DOI: 10.4062/biomolther.2016.263
Role of TAZ in Lysophosphatidic Acid-Induced Migration and Proliferation of Human Adipose-Derived Mesenchymal Stem Cells
Abstract
Transcriptional co-activator with a PDZ-binding motif (TAZ) is an important factor in lysophosphatidic acid (LPA)-induced promotion of migration and proliferation of human mesenchymal stem cells (MSCs). The expression of TAZ significantly increased at 6 h after LPA treatment, and TAZ knockdown inhibited the LPA-induced migration and proliferation of MSCs. In addition, embryonic fibroblasts from TAZ knockout mice exhibited the reduction in LPA-induced migration and proliferation. The LPA1 receptor inhibitor Ki16425 blocked LPA responses in MSCs. Although TAZ knockdown or knockout did not reduce LPA-induced phosphorylation of ERK and AKT, the MEK inhibitor U0126 or the ROCK inhibitor Y27632 blocked LPA-induced TAZ expression along with the reduction in the proliferation and migration of MSCs. Our data suggest that TAZ is an important mediator of LPA signaling in MSCs in the downstream of MEK and ROCK signaling.
Keywords: LPA; MEK; Mesenchymal stem cells; ROCK; TAZ.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

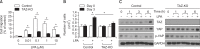


Similar articles
-
TAZ mediates lysophosphatidic acid-induced migration and proliferation of epithelial ovarian cancer cells.Cell Physiol Biochem. 2013;32(2):253-63. doi: 10.1159/000354434. Epub 2013 Jul 31. Cell Physiol Biochem. 2013. PMID: 23942151
-
Lysophosphatidic acid in malignant ascites stimulates migration of human mesenchymal stem cells.J Cell Biochem. 2008 May 15;104(2):499-510. doi: 10.1002/jcb.21641. J Cell Biochem. 2008. PMID: 18027882
-
IRS-1 targets TAZ to inhibit adipogenesis of rat bone marrow mesenchymal stem cells through PI3K-Akt and MEK-ERK pathways.Eur J Pharmacol. 2019 Apr 15;849:11-21. doi: 10.1016/j.ejphar.2019.01.064. Epub 2019 Feb 1. Eur J Pharmacol. 2019. PMID: 30716312
-
Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ.Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):21-6. doi: 10.1016/j.bbrc.2015.11.006. Epub 2015 Nov 6. Biochem Biophys Res Commun. 2015. PMID: 26549225
-
GPR87 mediates lysophosphatidic acid-induced colony dispersal in A431 cells.Eur J Pharmacol. 2013 Sep 5;715(1-3):15-20. doi: 10.1016/j.ejphar.2013.06.029. Epub 2013 Jul 4. Eur J Pharmacol. 2013. PMID: 23831392 Review.
Cited by
-
Alteration of Genomic Imprinting Status of Human Parthenogenetic Induced Pluripotent Stem Cells during Neural Lineage Differentiation.Int J Stem Cells. 2019 Mar 30;12(1):31-42. doi: 10.15283/ijsc18084. Int J Stem Cells. 2019. PMID: 30836722 Free PMC article.
-
Therapeutic Strategies for Targeting Ovarian Cancer Stem Cells.Int J Mol Sci. 2021 May 11;22(10):5059. doi: 10.3390/ijms22105059. Int J Mol Sci. 2021. PMID: 34064635 Free PMC article. Review.
-
YDJC Induces Epithelial-Mesenchymal Transition via Escaping from Interaction with CDC16 through Ubiquitination of PP2A.J Oncol. 2019 Aug 7;2019:3542537. doi: 10.1155/2019/3542537. eCollection 2019. J Oncol. 2019. PMID: 31485224 Free PMC article.
-
Role for CCN1 in lysophosphatidic acid response in PC-3 human prostate cancer cells.J Cell Commun Signal. 2024 Feb 20;18(1):e12019. doi: 10.1002/ccs3.12019. eCollection 2024 Mar. J Cell Commun Signal. 2024. PMID: 38545253 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

