Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study
- PMID: 28554238
- PMCID: PMC6029149
- DOI: 10.1177/1352458517709619
Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study
Abstract
Background: Confirmed Expanded Disability Status Scale (EDSS) progression occurring after a fixed-study entry baseline is a common measure of disability increase in relapsing-remitting multiple sclerosis (RRMS) studies but may not detect all disability progression events, especially those unrelated to overt relapses.
Objective: To evaluate possible measures of disability progression unrelated to relapse using EDSS data over ≈5.5 years from the Tysabri® Observational Program (TOP).
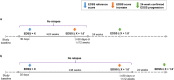
Methods: TOP is an ongoing, prospective, open-label study in RRMS patients receiving intravenous 300 mg natalizumab every 4 weeks. Measures of increasing disability were assessed using as a reference either study baseline score or a "roving" system that resets the reference score after ⩾24- or ⩾48-week confirmation of a new score.
Results: This analysis included 5562 patients. Approximately 70% more EDSS progression events unrelated to relapse and 50% more EDSS worsening events overall were detected with a roving reference score (cumulative probability: 17.6% and 29.7%, respectively) than with a fixed reference baseline score (cumulative probability: 10.1% and 20.3%, respectively).
Conclusion: In this long-term observational RRMS dataset, a roving EDSS reference value was more efficient than a study baseline EDSS reference in detecting progression/worsening events unrelated to relapses and thus the transition to secondary progressive disease.
Keywords: Expanded Disability Status Scale; Multiple sclerosis; disease progression; natalizumab; relapsing-remitting; secondary progressive multiple sclerosis.
Conflict of interest statement
Figures



References
-
- Rudick RA, Lee JC, Cutter GR, et al. Disability progression in a clinical trial of relapsing-remitting multiple sclerosis: Eight-year follow-up. Arch Neurol 2010; 67: 1329–1335. - PubMed
-
- Kalincik T, Cutter GR, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain 2015; 138: 3287–3298. - PubMed
-
- European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis, 2015, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (accessed 11 June 2015).
-
- Phillips JT, Giovannoni G, Lublin FD, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: Treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 2011; 17: 970–979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

