CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury
- PMID: 28554875
- PMCID: PMC5875702
- DOI: 10.1016/j.jhep.2017.05.021
CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury
Abstract
Background & aims: The severity of sepsis can be linked to excessive inflammatory responses resulting in hepatic injury. P2X7 receptor activation by extracellular ATP (eATP) exacerbates inflammation by augmenting cytokine production; while CD39 (ENTPD1) scavenges eATP to generate adenosine, thereby limiting P2X7 activation and resulting in A2A receptor stimulation. We aim to determine how the functional interaction of P2X7 receptor and CD39 control the macrophage response, and consequently impact on sepsis and liver injury.
Methods: Sepsis was induced by cecal ligation and puncture in C57BL/6 wild-type (WT) and CD39-/- mice. Several in vitro assays were performed using peritoneal or bone marrow derived macrophages to determine CD39 ectonucleotidase activity and its role in sepsis-induced liver injury.
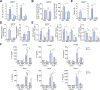
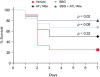
Results: CD39 expression in macrophages limits ATP-P2X7 receptor pro-inflammatory signaling. P2X7 receptor paradoxically boosts CD39 activity. Inhibition and/or deletion of P2X7 receptor in LPS-primed macrophages attenuates cytokine production and inflammatory signaling as well as preventing ATP-induced increases in CD39 activity. Septic CD39-/- mice exhibit higher levels of inflammatory cytokines and show more pronounced liver injury than WT mice. Pharmacological P2X7 blockade largely prevents tissue damage, cell apoptosis, cytokine production, and the activation of inflammatory signaling pathways in the liver from septic WT, while only attenuating these outcomes in CD39-/- mice. Furthermore, the combination of P2X7 blockade with adenosine A2A receptor stimulation completely inhibits cytokine production, the activation of inflammatory signaling pathways, and protects septic CD39-/- mice against liver injury.
Conclusions: CD39 attenuates sepsis-associated liver injury by scavenging eATP and ultimately generating adenosine. We propose boosting of CD39 would suppress P2X7 responses and trigger adenosinergic signaling to limit systemic inflammation and restore liver homeostasis during the acute phase of sepsis. Lay summary: CD39 expression in macrophages limits P2X7-mediated pro-inflammatory responses, scavenging extracellular ATP and ultimately generating adenosine. CD39 genetic deletion exacerbates sepsis-induced experimental liver injury. Combinations of a P2X7 antagonist and adenosine A2A receptor agonist are hepatoprotective during the acute phase of abdominal sepsis.
Keywords: Adenosine; Ectonucleotidases; Extracellular ATP; Kupffer cells; Systemic inflammation.
Copyright © 2017 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
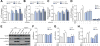





References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Possamai LA, Thursz MR, Wendon JA, Antoniades CG. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61:439–445. - PubMed
-
- Antoniades CG, Khamri W, Abeles RD, Taams LS, Triantafyllou E, Possamai LA, et al. Secretory leukocyte protease inhibitor: a pivotal mediator of anti-inflammatory responses in acetaminophen-induced acute liver failure. Hepatology. 2014;59:1564–1576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

