Development of Glutamatergic Proteins in Human Visual Cortex across the Lifespan
- PMID: 28554889
- PMCID: PMC6596503
- DOI: 10.1523/JNEUROSCI.2304-16.2017
Development of Glutamatergic Proteins in Human Visual Cortex across the Lifespan
Abstract
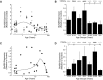
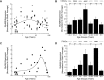
Traditionally, human primary visual cortex (V1) has been thought to mature within the first few years of life, based on anatomical studies of synapse formation, and establishment of intracortical and intercortical connections. Human vision, however, develops well beyond the first few years. Previously, we found prolonged development of some GABAergic proteins in human V1 (Pinto et al., 2010). Yet as >80% of synapses in V1 are excitatory, it remains unanswered whether the majority of synapses regulating experience-dependent plasticity and receptive field properties develop late, like their inhibitory counterparts. To address this question, we used Western blotting of postmortem tissue from human V1 (12 female, 18 male) covering a range of ages. Then we quantified a set of postsynaptic glutamatergic proteins (PSD-95, GluA2, GluN1, GluN2A, GluN2B), calculated indices for functional pairs that are developmentally regulated (GluA2:GluN1; GluN2A:GluN2B), and determined interindividual variability. We found early loss of GluN1, prolonged development of PSD-95 and GluA2 into late childhood, protracted development of GluN2A until ∼40 years, and dramatic loss of GluN2A in aging. The GluA2:GluN1 index switched at ∼1 year, but the GluN2A:GluN2B index continued to shift until ∼40 year before changing back to GluN2B in aging. We also identified young childhood as a stage of heightened interindividual variability. The changes show that human V1 develops gradually through a series of five orchestrated stages, making it likely that V1 participates in visual development and plasticity across the lifespan.SIGNIFICANCE STATEMENT Anatomical structure of human V1 appears to mature early, but vision changes across the lifespan. This discrepancy has fostered two hypotheses: either other aspects of V1 continue changing, or later changes in visual perception depend on extrastriate areas. Previously, we showed that some GABAergic synaptic proteins change across the lifespan, but most synapses in V1 are excitatory leaving unanswered how they change. So we studied expression of glutamatergic proteins in human V1 to determine their development. Here we report prolonged maturation of glutamatergic proteins, with five stages that map onto life-long changes in human visual perception. Thus, the apparent discrepancy between development of structure and function may be explained by life-long synaptic changes in human V1.
Keywords: development; glutamate; human; receptors; synaptic proteins; visual cortex.
Copyright © 2017 the authors 0270-6474/17/376031-12$15.00/0.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
