Fetuin-A aggravates lipotoxicity in podocytes via interleukin-1 signaling
- PMID: 28554965
- PMCID: PMC5449566
- DOI: 10.14814/phy2.13287
Fetuin-A aggravates lipotoxicity in podocytes via interleukin-1 signaling
Abstract
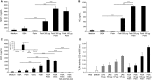
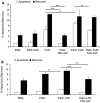
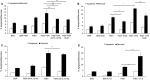
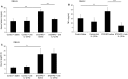
Sterile inflammation is considered critical in the pathogenesis of diabetic nephropathy (DN). Here we show that Fetuin-A (FetA) or lipopolysaccharide (LPS) exacerbate palmitic acid-induced podocyte death, which is associated with a strong induction of monocyte chemoattractant protein-1 (MCP-1) and keratinocyte chemoattractant (KC). Moreover, blockage of TLR4 prevents MCP-1 and KC secretion and attenuates podocyte death induced by palmitic acid alone or combined with FetA. In addition, inhibition of interleukin-1 (IL-1) signaling by anakinra, a recombinant human IL-1Ra, or a murinized anti-IL-1β antibody attenuates the inflammatory and ultimate cell death response elicited by FetA alone or combined with palmitic acid. In vivo short-term therapy of diabetic DBA/2J mice with an anti-IL1-β antibody for 4 weeks prevented an increase in serum FetA and considerably decreased urinary tumor necrosis alpha (TNF-α), a known risk factor for DN progression. In summary, our results suggest that FetA similarly to LPS leads to an inflammatory response in podocytes, which exacerbates palmitic acid-induced podocyte death and our data imply a critical role for IL-1β signaling in this process. The study offers the rational for prolonged in vivo studies aimed at testing anti-IL-1β therapy for prevention and treatment of DN.
Keywords: Diabetic nephropathy; Fetuin‐A; free fatty acids; interleukin‐1; palmitic acid; toll‐like receptor.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have no conflict of interests.
Figures

References
-
- Akira, S. , Takeda K., and Kaisho T.. 2001. Toll‐like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. - PubMed
-
- Blakemore, A. I. , Cox A., Gonzalez A. M., Maskil J. K., Hughes M. E., Wilson R. M., et al. 1996. Interleukin‐1 receptor antagonist allele (IL1RN*2) associated with nephropathy in diabetes mellitus. Hum. Genet. 97:369–374. - PubMed
-
- Boni‐Schnetzler, M. , Boller S., Debray S., Bouzakri K., Meier D. T., Prazak R., et al. 2009. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin‐1 receptor I. Endocrinology 150:5218–5229. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

