Recent Advances in the Development of Mammalian Geranylgeranyl Diphosphate Synthase Inhibitors
- PMID: 28555000
- PMCID: PMC5902023
- DOI: 10.3390/molecules22060886
Recent Advances in the Development of Mammalian Geranylgeranyl Diphosphate Synthase Inhibitors
Abstract
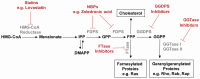
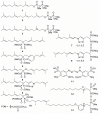
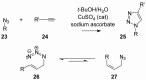
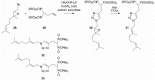
The enzyme geranylgeranyl diphosphate synthase (GGDPS) catalyzes the synthesis of the 20-carbon isoprenoid geranylgeranyl diphosphate (GGPP). GGPP is the isoprenoid donor for protein geranylgeranylation reactions catalyzed by the enzymes geranylgeranyl transferase (GGTase) I and II. Inhibitors of GGDPS result in diminution of protein geranylgeranylation through depletion of cellular GGPP levels, and there has been interest in GGDPS inhibitors as potential anti-cancer agents. Here we discuss recent advances in the development of GGDPS inhibitors, including insights gained by structure-function relationships, and review the preclinical data that support the continued development of this novel class of drugs.
Keywords: geranylgeranyl diphosphate synthase; geranylgeranylation; inhibitors; isoprenoid.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.F.W. is a named inventor of intellectual property related to digeranyl bisphosphonate that is owned by the University of Iowa Research Foundation. He is a founder of Terpenoid Therapeutics, Inc., which has licensed this property.
Figures

References
-
- Chao Y.S., Kroon P.A., Yamin T.T., Thompson G.M., Alberts A.W. Regulation of hepatic receptor-dependent degradation of LDL by mevinolin in rabbits with hypercholesterolemia induced by a wheat starch-casein diet. Biochim. Biophys. Acta. 1983;754:134–141. doi: 10.1016/0005-2760(83)90154-6. - DOI - PubMed
-
- Bilheimer D.W., Grundy S.M., Brown M.S., Goldstein J.L. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc. Natl. Acad. Sci. USA. 1983;80:4124–4128. doi: 10.1073/pnas.80.13.4124. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

