Recent Advances in Asymmetric Organocatalyzed Conjugate Additions to Nitroalkenes
- PMID: 28555049
- PMCID: PMC6152790
- DOI: 10.3390/molecules22060895
Recent Advances in Asymmetric Organocatalyzed Conjugate Additions to Nitroalkenes
Abstract
The asymmetric conjugate addition of carbon and heteroatom nucleophiles to nitroalkenes is a very interesting tool for the construction of highly functionalized synthetic building blocks. Thanks to the rapid development of asymmetric organocatalysis, significant progress has been made during the last years in achieving efficiently this process, concerning chiral organocatalysts, substrates and reaction conditions. This review surveys the advances in asymmetric organocatalytic conjugate addition reactions to α,β-unsaturated nitroalkenes developed between 2013 and early 2017.
Keywords: Michael addition; asymmetric synthesis; nitroalkenes; organocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
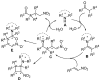

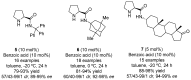



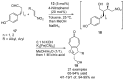
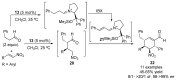



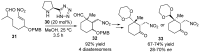

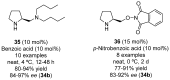



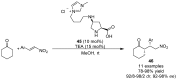

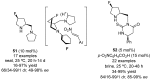



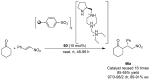



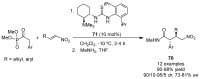

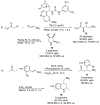
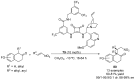
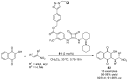
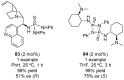
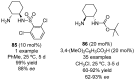
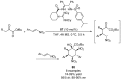


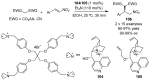
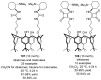
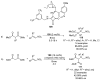

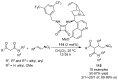
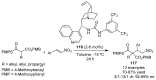


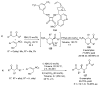




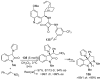





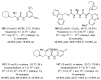






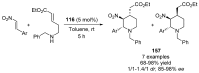

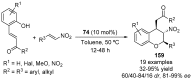

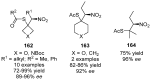




References
-
- Dalko P.I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications. Wiley-VCH; Weinheim, Germany: 2013.
-
- Atodiresei I., Vila C., Rueping M. Asymmetric organocatalysis in continuous flow: Opportunities for impacting industrial catalysis. ACS Catal. 2015;5:1972–1985. doi: 10.1021/acscatal.5b00002. - DOI
-
- Krause N., Hoffmann-Roder A. Recent advances in catalytic enantioselective Michael additions. Synthesis. 2001;2001:0171–0196. doi: 10.1055/s-2001-10803. - DOI
-
- Jha S.C., Joshi N.N. Catalytic, enantioselective Michael addition reactions. ARKIVOC. 2002;viii:167–196. doi: 10.1002/chin.200329238. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

