The Methods of Choice for Extracellular Vesicles (EVs) Characterization
- PMID: 28555055
- PMCID: PMC5485977
- DOI: 10.3390/ijms18061153
The Methods of Choice for Extracellular Vesicles (EVs) Characterization
Abstract
In recent years, extracellular vesicles (EVs) have become a subject of intense study. These membrane-enclosed spherical structures are secreted by almost every cell type and are engaged in the transport of cellular content (cargo) from parental to target cells. The impact of EVs transfer has been observed in many vital cellular processes including cell-to-cell communication and immune response modulation; thus, a fast and precise characterization of EVs may be relevant for both scientific and diagnostic purposes. In this review, the most popular analytical techniques used in EVs studies are presented with the emphasis on exosomes and microvesicles characterization.
Keywords: atomic force microscopy (AFM); cryo-electron microscopy (Cryo-EM); dynamic light scattering (DLS); exosomes; extracellular vesicles (EVs); flow cytometry; microvesicles (MVs); nanoparticle tracking analysis (NTA); stimulated emission depletion microscopy (STED); transmission electron microscopy (TEM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

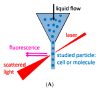

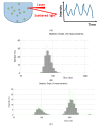



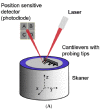

References
-
- Théry C., Zitvogel L., Amigorena S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

