Reduced Membrane Insertion of CLC-K by V33L Barttin Results in Loss of Hearing, but Leaves Kidney Function Intact
- PMID: 28555110
- PMCID: PMC5430073
- DOI: 10.3389/fphys.2017.00269
Reduced Membrane Insertion of CLC-K by V33L Barttin Results in Loss of Hearing, but Leaves Kidney Function Intact
Abstract
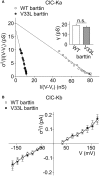
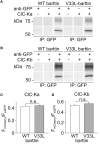
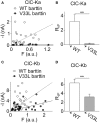
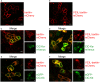
In the mammalian ear, transduction of sound stimuli is initiated by K+ entry through mechano-sensitive channels into inner hair cells. K+ entry is driven by a positive endocochlear potential that is maintained by the marginal cell layer of the stria vascularis. This process requires basolateral K+ import by NKCC1 Na+-2Cl--K+ co-transporters as well as Cl- efflux through ClC-Ka/barttin or ClC-Kb/barttin channels. Multiple mutations in the gene encoding the obligatory CLC-K subunit barttin, BSND, have been identified in patients with Bartter syndrome type IV. These mutations reduce the endocochlear potential and cause deafness. As CLC-K/barttin channels are also expressed in the kidney, patients with Bartter syndrome IV typically also suffer from salt-wasting hyperuria and electrolyte imbalances. However, there was a single report on a BSND mutation that resulted only in deafness, but not kidney disease. We herein studied the functional consequences of another recently discovered BSND mutation that predicts exchange of valine at position 33 by leucine. We combined whole-cell patch clamp, confocal microscopy and protein biochemistry to analyze how V33L affects distinct functions of barttin. We found that V33L reduced membrane insertion of CLC-K/barttin complexes without altering unitary CLC-K channel function. Our findings support the hypothesis of a common pathophysiology for the selective loss of hearing due to an attenuation of the total chloride conductance in the stria vascularis while providing enough residual function to maintain normal kidney function.
Keywords: Bartter syndrome; CLC channel; barttin; hearing loss; patch clamp.
Figures


Similar articles
-
Activation of renal ClC-K chloride channels depends on an intact N terminus of their accessory subunit barttin.J Biol Chem. 2018 Jun 1;293(22):8626-8637. doi: 10.1074/jbc.RA117.000860. Epub 2018 Apr 19. J Biol Chem. 2018. PMID: 29674316 Free PMC article.
-
Physiology and pathophysiology of ClC-K/barttin channels.Front Physiol. 2010 Nov 26;1:155. doi: 10.3389/fphys.2010.00155. eCollection 2010. Front Physiol. 2010. PMID: 21423394 Free PMC article.
-
Barttin increases surface expression and changes current properties of ClC-K channels.Pflugers Arch. 2002 Jun;444(3):411-8. doi: 10.1007/s00424-002-0819-8. Epub 2002 Apr 9. Pflugers Arch. 2002. PMID: 12111250
-
Bartter syndrome.Curr Opin Nephrol Hypertens. 2003 Sep;12(5):527-32. doi: 10.1097/00041552-200309000-00008. Curr Opin Nephrol Hypertens. 2003. PMID: 12920401 Review.
-
Mechanisms of Disease: the kidney-specific chloride channels ClCKA and ClCKB, the Barttin subunit, and their clinical relevance.Nat Clin Pract Nephrol. 2008 Jan;4(1):38-46. doi: 10.1038/ncpneph0689. Nat Clin Pract Nephrol. 2008. PMID: 18094726 Review.
Cited by
-
Vesicular glutamate transporters are H+-anion exchangers that operate at variable stoichiometry.Nat Commun. 2023 May 11;14(1):2723. doi: 10.1038/s41467-023-38340-9. Nat Commun. 2023. PMID: 37169755 Free PMC article.
-
Reconstitution and NMR Characterization of the Ion-Channel Accessory Subunit Barttin in Detergents and Lipid-Bilayer Nanodiscs.Front Mol Biosci. 2019 Mar 14;6:13. doi: 10.3389/fmolb.2019.00013. eCollection 2019. Front Mol Biosci. 2019. PMID: 30931313 Free PMC article.
-
Functional Study of Novel Bartter's Syndrome Mutations in ClC-Kb and Rescue by the Accessory Subunit Barttin Toward Personalized Medicine.Front Pharmacol. 2020 Mar 17;11:327. doi: 10.3389/fphar.2020.00327. eCollection 2020. Front Pharmacol. 2020. PMID: 32256370 Free PMC article.
-
Dual-color Colocalization in Single-molecule Localization Microscopy to Determine the Oligomeric State of Proteins in the Plasma Membrane.Bio Protoc. 2023 Jul 5;13(13):e4749. doi: 10.21769/BioProtoc.4749. eCollection 2023 Jul 5. Bio Protoc. 2023. PMID: 37456335 Free PMC article.
-
Determination of oligomeric states of proteins via dual-color colocalization with single molecule localization microscopy.Elife. 2022 Oct 7;11:e76631. doi: 10.7554/eLife.76631. Elife. 2022. PMID: 36205395 Free PMC article.
References
-
- Cappola T. P., Matkovich S. J., Wang W., Van Booven D., Li M., Wang X., et al. . (2011). Loss-of-function DNA sequence variant in the CLCNKA chloride channel implicates the cardio-renal axis in interindividual heart failure risk variation. Proc. Natl. Acad. Sci. U.S.A. 108, 2456–2461. 10.1073/pnas.1017494108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

