Caveolin-1: Functional Insights into Its Role in Muscarine- and Serotonin-Induced Smooth Muscle Constriction in Murine Airways
- PMID: 28555112
- PMCID: PMC5430063
- DOI: 10.3389/fphys.2017.00295
Caveolin-1: Functional Insights into Its Role in Muscarine- and Serotonin-Induced Smooth Muscle Constriction in Murine Airways
Abstract
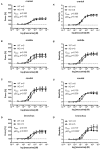
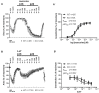
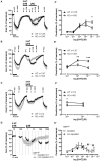
An increased bronchoconstrictor response is a hallmark in the progression of obstructive airway diseases. Acetylcholine and 5-hydroxytryptamine (5-HT, serotonin) are the major bronchoconstrictors. There is evidence that both cholinergic and serotonergic signaling in airway smooth muscle (ASM) involve caveolae. We hypothesized that caveolin-1 (cav-1), a structural protein of caveolae, plays an important regulatory role in ASM contraction. We analyzed airway contraction in different tracheal segments and extra- and intrapulmonary bronchi in cav-1 deficient (cav-1-/-) and wild-type mice using organ bath recordings and videomorphometry of methyl-beta-cyclodextrin (MCD) treated and non-treated precision-cut lung slices (PCLS). The presence of caveolae was investigated by electron microscopy. Receptor subtypes driving 5-HT-responses were studied by RT-PCR and videomorphometry after pharmacological inhibition with ketanserin. Cav-1 was present in tracheal epithelium and ASM. Muscarine induced a dose dependent contraction in all airway segments. A significantly higher Emax was observed in the caudal trachea. Although, caveolae abundancy was largely reduced in cav-1-/- mice, muscarine-induced airway contraction was maintained, albeit at diminished potency in the middle trachea, in the caudal trachea and in the bronchus without changes in the maximum efficacy. MCD-treatment of PLCS from cav-1-/- mice reduced cholinergic constriction by about 50%, indicating that cholesterol-rich plasma domains account for a substantial portion of the muscarine-induced bronchoconstriction. Notably, cav-1-deficiency fully abrogated 5-HT-induced contraction of extrapulmonary airways. In contrast, 5-HT-induced bronchoconstriction was fully maintained in cav-1-deficient intrapulmonary bronchi, but desensitization upon repetitive stimulation was enhanced. RT-PCR analysis revealed 5-HT1B, 5-HT2A, 5-HT6, and 5-HT7 receptors as the most prevalent subtypes in the airways. The 5-HT-induced-constriction in PCLS could be antagonized by ketanserin, a 5-HT2A receptor inhibitor. In conclusion, the role of cav-1, caveolae, and cholesterol-rich plasma domains in regulation of airway tone are highly agonist-specific and dependent on airway level. Cav-1 is indispensable for serotonergic contraction of extrapulmonary airways and modulates cholinergic constriction of the trachea and main bronchus. Thus, cav-1/caveolae shall be considered in settings such as bronchial hyperreactivity in common airway diseases and might provide an opportunity for modulation of the constrictor response.
Keywords: 5-HT; airway smooth muscle; bronchus; caveolin-1; contraction; muscarine.
Figures







Similar articles
-
Caveolin-3 differentially orchestrates cholinergic and serotonergic constriction of murine airways.Sci Rep. 2018 May 14;8(1):7508. doi: 10.1038/s41598-018-25445-1. Sci Rep. 2018. PMID: 29760450 Free PMC article.
-
Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse.Respir Res. 2006 Apr 12;7(1):65. doi: 10.1186/1465-9921-7-65. Respir Res. 2006. PMID: 16608531 Free PMC article.
-
Muscarinic receptor-mediated bronchoconstriction is coupled to caveolae in murine airways.Am J Physiol Lung Cell Mol Physiol. 2010 May;298(5):L626-36. doi: 10.1152/ajplung.00261.2009. Epub 2009 Dec 18. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20023174 Free PMC article.
-
Regulation of Airway Smooth Muscle Contraction in Health and Disease.Adv Exp Med Biol. 2019;1124:381-422. doi: 10.1007/978-981-13-5895-1_16. Adv Exp Med Biol. 2019. PMID: 31183836 Review.
-
Role of the parasympathetic nervous system and of cholinergic mechanisms in bronchial hyperreactivity.Bull Eur Physiopathol Respir. 1986;22 Suppl 7:112-42. Bull Eur Physiopathol Respir. 1986. PMID: 3006841 Review.
Cited by
-
Altered Lipid Domains Facilitate Enhanced Pulmonary Vasoconstriction after Chronic Hypoxia.Am J Respir Cell Mol Biol. 2020 Jun;62(6):709-718. doi: 10.1165/rcmb.2018-0318OC. Am J Respir Cell Mol Biol. 2020. PMID: 31945301 Free PMC article.
-
5-HTP inhibits eosinophilia via intracellular endothelial 5-HTRs; SNPs in 5-HTRs associate with asthmatic lung function.Front Allergy. 2024 May 23;5:1385168. doi: 10.3389/falgy.2024.1385168. eCollection 2024. Front Allergy. 2024. PMID: 38845678 Free PMC article.
-
Sertraline-Induced Acute Eosinophilic Pneumonia.Cureus. 2020 Dec 11;12(12):e12022. doi: 10.7759/cureus.12022. Cureus. 2020. PMID: 33457126 Free PMC article.
-
Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development.Int J Mol Sci. 2023 Feb 3;24(3):2965. doi: 10.3390/ijms24032965. Int J Mol Sci. 2023. PMID: 36769290 Free PMC article.
-
Differential targeting and signalling of voltage-gated T-type Cav 3.2 and L-type Cav 1.2 channels to ryanodine receptors in mesenteric arteries.J Physiol. 2018 Oct;596(20):4863-4877. doi: 10.1113/JP276923. Epub 2018 Sep 15. J Physiol. 2018. PMID: 30146760 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

