Viruses and miRNAs: More Friends than Foes
- PMID: 28555130
- PMCID: PMC5430039
- DOI: 10.3389/fmicb.2017.00824
Viruses and miRNAs: More Friends than Foes
Abstract
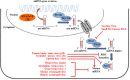
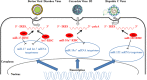
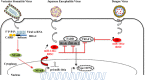
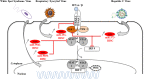
There is evidence that eukaryotic miRNAs (hereafter called host miRNAs) play a role in the replication and propagation of viruses. Expression or targeting of host miRNAs can be involved in cellular antiviral responses. Most times host miRNAs play a role in viral life-cycles and promote infection through complex regulatory pathways. miRNAs can also be encoded by a viral genome and be expressed in the host cell. Viral miRNAs can share common sequences with host miRNAs or have totally different sequences. They can regulate a variety of biological processes involved in viral infection, including apoptosis, evasion of the immune response, or modulation of viral life-cycle phases. Overall, virus/miRNA pathway interaction is defined by a plethora of complex mechanisms, though not yet fully understood. This article review summarizes recent advances and novel biological concepts related to the understanding of miRNA expression, control and function during viral infections. The article also discusses potential therapeutic applications of this particular host-pathogen interaction.
Keywords: clinical perspectives; gene expression; microRNAs; viral microRNAs; virus life cycle.
Figures
References
-
- Abend J. R., Ramalingam D., Kieffer-Kwon P., Uldrick T. S., Yarchoan R., Ziegelbauer J. M. (2012). Kaposi’s sarcoma-associated herpesvirus microRNAs target IRAK1 and MYD88, two components of the toll-like receptor/interleukin-1R signaling cascade, to reduce inflammatory-cytokine expression. J. Virol. 86 11663–11674. 10.1128/JVI.01147-12 - DOI - PMC - PubMed
-
- Abend J. R., Uldrick T., Ziegelbauer J. M. (2010). Regulation of tumor necrosis factor-like weak inducer of apoptosis receptor protein (TWEAKR) expression by Kaposi’s sarcoma-associated herpesvirus microRNA prevents TWEAK-induced apoptosis and inflammatory cytokine expression. J. Virol. 84 12139–12151. 10.1128/JVI.00884-10 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

