The P2Y1 receptor-mediated leukocyte adhesion to endothelial cells is inhibited by melatonin
- PMID: 28555330
- PMCID: PMC5563294
- DOI: 10.1007/s11302-017-9565-4
The P2Y1 receptor-mediated leukocyte adhesion to endothelial cells is inhibited by melatonin
Abstract
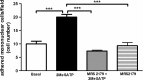
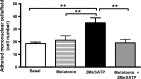
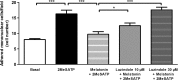
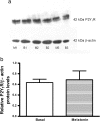
Extracellular ATP (released by endothelial and immune cells) and its metabolite ADP are important pro-inflammatory mediators via the activation of purinergic P2 receptors (P2Y and P2X), which represent potential new targets for anti-inflammatory therapy. Endothelial P2Y1 receptor (P2Y1R) induces endothelial cell activation triggering leukocyte adhesion. A number of data have implicated melatonin as a modulator of immunity, inflammation, and endothelial cell function, but to date no studies have investigated whether melatonin modulates endothelial P2YR signaling. Here, we evaluated the putative effect of melatonin on P2Y1R-mediated leukocyte adhesion to endothelial cells and TNF-α production, using mesenteric endothelial cells and fresh peripheral blood mononuclear cells isolated from rats. Endothelial cells were treated with the P2Y1R agonist 2MeSATP, alone or in combination with melatonin, and then exposed to mononuclear cells. 2MeSATP increased leukocyte adhesion to endothelial cells and TNF-α production in vitro, and melatonin inhibited both effects without altering P2Y1R protein expression. In addition, assays with the Ca2+ chelator BAPTA-AM indicate that the effect of melatonin on 2MeSATP-stimulated leukocyte adhesion depends on intracellular Ca2+ modulation. P2Y1R is considered a potential target to control chronic inflammation. Therefore, our data unveiled a new endothelial cell modulator of purinergic P2Y1 receptor signaling.
Keywords: Endothelial cells; Inflammation; Melatonin; P2Y1 receptor; Purinergic signaling.
Conflict of interest statement
Funding
This study was funded by National Council for Scientific and Technological Development (CNPq, Brazil, grant number 455436/2014-2).
Conflict of interest
Tassya Cataldi Cardoso declares that she has no conflict of interest.
Thaís Emanuelle Pompeu declares that she has no conflict of interest.
Claudia Lucia Martins Silva declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

