Central and peripheral clocks are coupled by a neuropeptide pathway in Drosophila
- PMID: 28555616
- PMCID: PMC5459987
- DOI: 10.1038/ncomms15563
Central and peripheral clocks are coupled by a neuropeptide pathway in Drosophila
Abstract
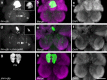
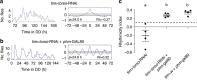
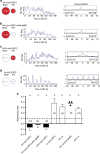
Animal circadian clocks consist of central and peripheral pacemakers, which are coordinated to produce daily rhythms in physiology and behaviour. Despite its importance for optimal performance and health, the mechanism of clock coordination is poorly understood. Here we dissect the pathway through which the circadian clock of Drosophila imposes daily rhythmicity to the pattern of adult emergence. Rhythmicity depends on the coupling between the brain clock and a peripheral clock in the prothoracic gland (PG), which produces the steroid hormone, ecdysone. Time information from the central clock is transmitted via the neuropeptide, sNPF, to non-clock neurons that produce the neuropeptide, PTTH. These secretory neurons then forward time information to the PG clock. We also show that the central clock exerts a dominant role on the peripheral clock. This use of two coupled clocks could serve as a paradigm to understand how daily steroid hormone rhythms are generated in animals.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Takeda N. & Maemura K. Circadian clock and vascular disease. Hypertens. Res. 33, 645–651 (2010). - PubMed
-
- Tsang A. H., Astiz M., Friedrichs M. & Oster H. Endocrine regulation of circadian physiology. J. Endocrinol. 230, R1–R11 (2016). - PubMed
-
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74, 246–260 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

