TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension
- PMID: 28555642
- PMCID: PMC5459967
- DOI: 10.1038/ncomms15494
TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension
Abstract
Pulmonary arterial hypertension (PAH) is an obstructive disease of the precapillary pulmonary arteries. Schistosomiasis-associated PAH shares altered vascular TGF-β signalling with idiopathic, heritable and autoimmune-associated etiologies; moreover, TGF-β blockade can prevent experimental pulmonary hypertension (PH) in pre-clinical models. TGF-β is regulated at the level of activation, but how TGF-β is activated in this disease is unknown. Here we show TGF-β activation by thrombospondin-1 (TSP-1) is both required and sufficient for the development of PH in Schistosoma-exposed mice. Following Schistosoma exposure, TSP-1 levels in the lung increase, via recruitment of circulating monocytes, while TSP-1 inhibition or knockout bone marrow prevents TGF-β activation and protects against PH development. TSP-1 blockade also prevents the PH in a second model, chronic hypoxia. Lastly, the plasma concentration of TSP-1 is significantly increased in subjects with scleroderma following PAH development. Targeting TSP-1-dependent activation of TGF-β could thus be a therapeutic approach in TGF-β-dependent vascular diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




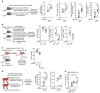

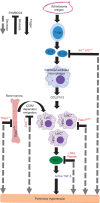
References
-
- Simonneau G. et al.. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 62, D34–D41 (2013). - PubMed
-
- Richter A. et al.. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 170, 1340–1348 (2004). - PubMed
-
- Lane K. B. et al.. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat. Genet. 26, 81–84 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

