A role for primary cilia in aortic valve development and disease
- PMID: 28556366
- PMCID: PMC5548133
- DOI: 10.1002/dvdy.24524
A role for primary cilia in aortic valve development and disease
Abstract
Background: Bicuspid aortic valve (BAV) disease is the most common congenital heart defect, affecting 0.5-1.2% of the population and causing significant morbidity and mortality. Only a few genes have been identified in pedigrees, and no single gene model explains BAV inheritance, thus supporting a complex genetic network of interacting genes. However, patients with rare syndromic diseases that stem from alterations in the structure and function of primary cilia ("ciliopathies") exhibit BAV as a frequent cardiovascular finding, suggesting primary cilia may factor broadly in disease etiology.
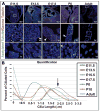
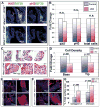
Results: Our data are the first to demonstrate that primary cilia are expressed on aortic valve mesenchymal cells during embryonic development and are lost as these cells differentiate into collagen-secreting fibroblastic-like cells. The function of primary cilia was tested by genetically ablating the critical ciliogenic gene Ift88. Loss of Ift88 resulted in abrogation of primary cilia and increased fibrogenic extracellular matrix (ECM) production. Consequentially, stratification of ECM boundaries normally present in the aortic valve were lost and a highly penetrant BAV phenotype was evident at birth.
Conclusions: Our data support cilia as a novel cellular mechanism for restraining ECM production during aortic valve development and broadly implicate these structures in the etiology of BAV disease in humans. Developmental Dynamics 246:625-634, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: bicuspid aortic valve; cardiac development; extracellular matrix; primary cilia.
© 2017 Wiley Periodicals, Inc.
Figures




References
-
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. - PubMed
-
- Broekhuis JR, Leong WY, Jansen G. Regulation of cilium length and intraflagellar transport. Int Rev Cell Mol Biol. 2013;303:101–138. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

