Drug sensitivity and resistance testing identifies PLK1 inhibitors and gemcitabine as potent drugs for malignant peripheral nerve sheath tumors
- PMID: 28556483
- PMCID: PMC5579334
- DOI: 10.1002/1878-0261.12086
Drug sensitivity and resistance testing identifies PLK1 inhibitors and gemcitabine as potent drugs for malignant peripheral nerve sheath tumors
Abstract
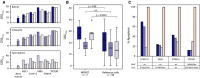
Patients with malignant peripheral nerve sheath tumor (MPNST), a rare soft tissue cancer associated with loss of the tumor suppressor neurofibromin (NF1), have poor prognosis and typically respond poorly to adjuvant therapy. We evaluated the effect of 299 clinical and investigational compounds on seven MPNST cell lines, two primary cultures of human Schwann cells, and five normal bone marrow aspirates, to identify potent drugs for MPNST treatment with few side effects. Top hits included Polo-like kinase 1 (PLK1) inhibitors (volasertib and BI2536) and the fluoronucleoside gemcitabine, which were validated in orthogonal assays measuring viability, cytotoxicity, and apoptosis. DNA copy number, gene expression, and protein expression were determined for the cell lines to assess pharmacogenomic relationships. MPNST cells were more sensitive to BI2536 and gemcitabine compared to a reference set of 94 cancer cell lines. PLK1, RRM1, and RRM2 mRNA levels were increased in MPNST compared to benign neurofibroma tissue, and the protein level of PLK1 was increased in the MPNST cell lines compared to normal Schwann cells, indicating an increased dependence on these drug targets in malignant cells. Furthermore, we observed an association between increased mRNA expression of PLK1, RRM1, and RRM2 in patient samples and worse disease outcome, suggesting a selective benefit from inhibition of these genes in the most aggressive tumors.
Keywords: MPNST; Schwann cell; drug screen; pharmacology.
© 2017 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures


References
-
- Abbou S, Lanvers‐Kaminsky C, Daudigeos‐Dubus E, LE Dret L, Laplace‐Builhe C, Molenaar J, Vassal G, Geoerger B, within the IB Preclinical Evaluation C (2016) Polo‐like kinase inhibitor volasertib exhibits antitumor activity and synergy with vincristine in pediatric malignancies. Anticancer Res 36, 599–609. - PubMed
-
- Ågesen TH, Flørenes VA, Molenaar WM, Lind GE, Berner JM, Plaat BE, Komdeur R, Myklebost O, van den Berg E and Lothe RA (2005) Expression patterns of cell cycle components in sporadic and neurofibromatosis type 1‐related malignant peripheral nerve sheath tumors. J Neuropathol Exp Neurol 64, 74–81. - PubMed
-
- Albritton KH, Rankin C, Coffin CM, Ratner N, Budd GT, Schuetze SM, Randall RL, Declue JE and Borden EC (2006) Phase II study of erlotinib in metastatic or unresectable malignant peripheral nerve sheath tumors (MPNST). J Clin Oncol 24, S9518.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

