Sodium intake and multiple sclerosis activity and progression in BENEFIT
- PMID: 28556498
- PMCID: PMC5555227
- DOI: 10.1002/ana.24965
Sodium intake and multiple sclerosis activity and progression in BENEFIT
Abstract
Objective: To assess whether a high-salt diet, as measured by urinary sodium concentration, is associated with faster conversion from clinically isolated syndrome (CIS) to multiple sclerosis (MS) and MS activity and disability.
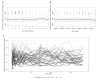
Methods: BENEFIT was a randomized clinical trial comparing early versus delayed interferon beta-1b treatment in 465 patients with a CIS. Each patient provided a median of 14 (interquartile range = 13-16) spot urine samples throughout the 5-year follow-up. We estimated 24-hour urine sodium excretion level at each time point using the Tanaka equations, and assessed whether sodium levels estimated from the cumulative average of the repeated measures were associated with clinical (conversion to MS, Expanded Disability Status Scale [EDSS]) and magnetic resonance imaging (MRI) outcomes.
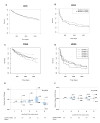
Results: Average 24-hour urine sodium levels were not associated with conversion to clinically definite MS over the 5-year follow-up (hazard ratio [HR] = 0.91, 95% confidence interval [CI] = 0.67-1.24 per 1g increase in estimated daily sodium intake), nor were they associated with clinical or MRI outcomes (new active lesions after 6 months: HR = 1.05, 95% CI = 0.97-1.13; relative change in T2 lesion volume: -0.11, 95% CI = -0.25 to 0.04; change in EDSS: -0.01, 95% CI = -0.09 to 0.08; relapse rate: HR = 0.78, 95% CI = 0.56-1.07). Results were similar in categorical analyses using quintiles.
Interpretation: Our results, based on multiple assessments of urine sodium excretion over 5 years and standardized clinical and MRI follow-up, suggest that salt intake does not influence MS disease course or activity. Ann Neurol 2017;82:20-29.
© 2017 American Neurological Association.
Conflict of interest statement
Figures

Comment in
-
Stratified analyses are necessary to verify the influence of salt intake in multiple sclerosis.Ann Neurol. 2017 Oct;82(4):648-649. doi: 10.1002/ana.25057. Ann Neurol. 2017. PMID: 28961329 No abstract available.
-
Reply to "Stratified analyses are necessary to verify the influence of salt intake in multiple sclerosis".Ann Neurol. 2017 Oct;82(4):649. doi: 10.1002/ana.25055. Epub 2017 Oct 10. Ann Neurol. 2017. PMID: 28961335 No abstract available.
References
-
- Ascherio A. Environmental factors in multiple sclerosis. Expert Rev Neurother. 2013;13:3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

