Hydrophilic interaction anion exchange for separation of multiply modified neutral and anionic Dictyostelium N-glycans
- PMID: 28556908
- PMCID: PMC5761722
- DOI: 10.1002/elps.201700073
Hydrophilic interaction anion exchange for separation of multiply modified neutral and anionic Dictyostelium N-glycans
Abstract
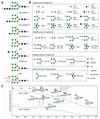
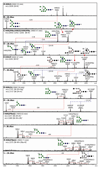
The unusual nature of the N-glycans of the cellular slime mould Dictyostelium discoideum has been revealed by a number of studies, primarily based on examination of radiolabeled glycopeptides but more recently also by MS. The complexity of the N-glycomes of even glycosylation mutants is compounded by the occurrence of anionic modifications, which also present an analytical challenge. In this study, we have employed hydrophilic interaction anion exchange (HIAX) HPLC in combination with MALDI-TOF MS/MS to explore the anionic N-glycome of the M31 (modA) strain, which lacks endoplasmic reticulum α-glucosidase II, an enzyme conserved in most eukaryotes including Homo sapiens. Prefractionation with HIAX chromatography enabled the identification of N-glycans with unusual oligo-α1,2-mannose extensions as well as others with up to four anionic modifications. Due to the use of hydrofluoric acid treatment, we were able to discriminate isobaric glycans differing in the presence of sulphate or phosphate on intersected structures as opposed to those carrying GlcNAc-phosphodiesters. The latter represent biosynthetic intermediates during the pathway leading to formation of the methylphosphorylated mannose epitope, which may have a similar function in intracellular targeting of hydrolases as the mannose-6-phosphate modification of lysosomal enzymes in mammals. In conclusion, HIAX in combination with MS is a highly sensitive approach for both fine separation and definition of neutral and anionic N-glycan structures.
Keywords: Glycome; HPLC; Mass spectrometry; Phosphate; Sulphate.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have no financial/commercial conflicts of interest.
Figures



Similar articles
-
N-glycomic profiling of a glucosidase II mutant of Dictyostelium discoideum by ''off-line'' liquid chromatography and mass spectrometry.Electrophoresis. 2014 Aug;35(15):2116-29. doi: 10.1002/elps.201300612. Epub 2014 Mar 31. Electrophoresis. 2014. PMID: 24574058 Free PMC article.
-
Mass spectrometric analysis of neutral and anionic N-glycans from a Dictyostelium discoideum model for human congenital disorder of glycosylation CDG IL.J Proteome Res. 2013 Mar 1;12(3):1173-87. doi: 10.1021/pr300806b. Epub 2013 Jan 27. J Proteome Res. 2013. PMID: 23320427 Free PMC article.
-
Analysis of Invertebrate and Protist N-Glycans.Methods Mol Biol. 2017;1503:167-184. doi: 10.1007/978-1-4939-6493-2_13. Methods Mol Biol. 2017. PMID: 27743366 Free PMC article.
-
Analysis of zwitterionic and anionic N-linked glycans from invertebrates and protists by mass spectrometry.Glycoconj J. 2016 Jun;33(3):273-83. doi: 10.1007/s10719-016-9650-x. Epub 2016 Feb 22. Glycoconj J. 2016. PMID: 26899268 Free PMC article. Review.
-
[Applications of chromatography in glycomics].Se Pu. 2024 Jul;42(7):646-657. doi: 10.3724/SP.J.1123.2023.12003. Se Pu. 2024. PMID: 38966973 Free PMC article. Review. Chinese.
Cited by
-
Anionic and zwitterionic moieties as widespread glycan modifications in non-vertebrates.Glycoconj J. 2020 Feb;37(1):27-40. doi: 10.1007/s10719-019-09874-2. Epub 2019 Jul 5. Glycoconj J. 2020. PMID: 31278613 Free PMC article. Review.
-
Towards understanding the extensive diversity of protein N-glycan structures in eukaryotes.Biol Rev Camb Philos Soc. 2022 Apr;97(2):732-748. doi: 10.1111/brv.12820. Epub 2021 Dec 6. Biol Rev Camb Philos Soc. 2022. PMID: 34873817 Free PMC article. Review.
-
Negative-mode mass spectrometry in the analysis of invertebrate, fungal, and protist N-glycans.Mass Spectrom Rev. 2022 Nov;41(6):945-963. doi: 10.1002/mas.21693. Epub 2021 May 6. Mass Spectrom Rev. 2022. PMID: 33955035 Free PMC article. Review.
-
Protein-Specific Analysis of Invertebrate Glycoproteins.Methods Mol Biol. 2019;1871:421-435. doi: 10.1007/978-1-4939-8814-3_24. Methods Mol Biol. 2019. PMID: 30276752 Free PMC article.
-
New insights into the N-glycomes of Dictyostelium species.BBA Adv. 2025 Jan 16;7:100142. doi: 10.1016/j.bbadva.2025.100142. eCollection 2025. BBA Adv. 2025. PMID: 39911813 Free PMC article.
References
-
- Urushihara H. The cellular slime mold: eukaryotic model microorganism. Exp Anim. 2009;58:97–104. - PubMed
-
- Freeze HH, Willies L, Hamilton S, Koza-Taylor P. Two mutants of Dictyostelium discoideum that lack a sulfated carbohydrate antigenic determinant synthesize a truncated lipid-linked precursor of N-linked oligosaccharides. J Biol Chem. 1989;264:5653–9. - PubMed
-
- Freeze HH, Wolgast D. Structural analysis of N-linked oligosaccharides from glycoproteins secreted by Dictyostelium discoideum. Identification of mannose 6-sulfate. J Biol Chem. 1986;261:127–34. - PubMed
-
- Couso R, van Halbeek H, Reinhold V, Kornfeld S. The high mannose oligosaccharides of Dictyostelium discoideum glycoproteins contain a novel intersecting N-acetylglucosamine residue. J Biol Chem. 1987;262:4521–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

