Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials
- PMID: 28556917
- PMCID: PMC5610607
- DOI: 10.1002/cncr.30791
Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials
Abstract
Background: Outcomes for children with acute myeloid leukemia (AML) have improved over the past 20 years even though the medications used for induction therapy have not changed.
Methods: This study analyzed data from patients with AML who were enrolled in successive protocols (AML97 and AML02) to determine the contributors to the improved outcomes of the latter clinical trial.
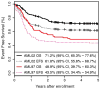
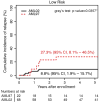
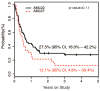
Results: There were significant improvements in 5-year overall survival (48.9% vs 71.2%; P < .0001) and event-free survival (43.5% vs 61.8%; P = .002) from AML97 to AML02. The 5-year cumulative incidence of early death (ED)/treatment-related mortality (TRM) was reduced for patients treated in AML02 (18.5% vs 7.9%; P = .007). Although the overall incidence of refractory disease (6.5% vs 5.6%; P = .736) and relapse (29.3% vs 21.0%; P = .12) did not differ between the 2 studies, patients with low-risk AML who were treated in AML02 had a reduced incidence of relapse (27.3% vs 8.8%; P = .036).
Conclusions: The improved outcomes of the AML02 trial resulted from improved disease control for low-risk patients and overall decreased ED/TRM. These results emphasize the importance of supportive-care measures throughout chemotherapy courses and hematopoietic cell transplantation and the value of treatment intensity for patients with low-risk AML while underscoring the need for novel therapy, rather than increased therapy intensity, for children with high-risk AML. Cancer 2017;123:3791-3798. © 2017 American Cancer Society.
Keywords: leukemia; outcomes; pediatric; supportive care.
© 2017 American Cancer Society.
Figures








References
-
- Kaplan E, Meier P. Non-parametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481.
-
- Link CL. Confidence intervals for the survival function using Cox's proportional-hazard model with covariates. Biometrics. 1984;40:601–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

