Treatment of spontaneous preterm labour with retosiban: a phase II pilot dose-ranging study
- PMID: 28556962
- PMCID: PMC5595955
- DOI: 10.1111/bcp.13336
Treatment of spontaneous preterm labour with retosiban: a phase II pilot dose-ranging study
Abstract
Aims: The aims of the present study were to investigate the maternal, fetal and neonatal safety and tolerability, pharmacodynamics and pharmacokinetics of intravenous (IV) retosiban in pregnant women with spontaneous preterm labour (PTL) between 340/7 and 356/7 weeks' gestation.
Methods: In parts A and B of a three-part, double-blind, placebo-controlled, multicentre study, women were randomized 3:1 (Part A) or 2:1 (Part B) to either 12-h IV retosiban followed by a single dose of oral placebo (R-P) or 12-h IV placebo followed by single-dose oral retosiban (P-R).
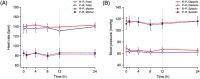
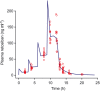
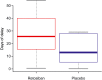
Results: A total of 29 women were randomized; 20 to R-P and nine to P-R. An integrated analysis found that adverse events were infrequent in mothers/newborns and consistent with events expected in the population under study or associated with confounding factors. Retosiban was rapidly absorbed after oral administration, with an observed half-life of 1.45 h. Efficacy analyses included 19 women. While not statistically significant, those receiving R-P more frequently achieved uterine quiescence in 6 h (R-P, 63%; 95% credible interval [CrI]: 38, 84; P-R, 43%; 95% CrI: 12, 78) and more achieved a reduction of ≥50% in uterine contractions in 6 h (R-P, 63%; 95% CrI: 38, 84; P-R, 29%; 95% CrI: 4, 64). The number of days to delivery was increased in women receiving R-P (median 26 days for R-P vs. 13 days for P-R).
Conclusions: Intravenous retosiban has a favourable safety and tolerability profile and might prolong pregnancies in women with PTL. The study provides the rationale and dosing strategy for further evaluation of the efficacy of retosiban in the treatment of PTL.
Keywords: bioavailability; pregnancy; randomized control trial.
© 2017 GlaxoSmithKline. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
Similar articles
-
Treatment of spontaneous preterm labour with retosiban: a phase 2 proof-of-concept study.Br J Clin Pharmacol. 2015 Oct;80(4):740-9. doi: 10.1111/bcp.12646. Epub 2015 Jun 1. Br J Clin Pharmacol. 2015. PMID: 25819462 Free PMC article. Clinical Trial.
-
Randomized Trials of Retosiban Versus Placebo or Atosiban in Spontaneous Preterm Labor.Am J Perinatol. 2021 Aug;38(S 01):e309-e317. doi: 10.1055/s-0040-1710034. Epub 2020 May 7. Am J Perinatol. 2021. PMID: 32380566 Clinical Trial.
-
Prevention of preterm delivery with vaginal progesterone in women with preterm labour (4P): randomised double-blind placebo-controlled trial.BJOG. 2015 Jan;122(1):80-91. doi: 10.1111/1471-0528.13061. Epub 2014 Sep 11. BJOG. 2015. PMID: 25209926 Clinical Trial.
-
Novel oxytocin receptor antagonists for tocolysis: a systematic review and meta-analysis of the available data on the efficacy, safety, and tolerability of retosiban.Curr Med Res Opin. 2021 Sep;37(9):1677-1688. doi: 10.1080/03007995.2021.1944076. Epub 2021 Jun 28. Curr Med Res Opin. 2021. PMID: 34134590
-
Prevention of preterm delivery.N Engl J Med. 2007 Aug 2;357(5):477-87. doi: 10.1056/NEJMra050435. N Engl J Med. 2007. PMID: 17671256 Review. No abstract available.
Cited by
-
Activation of oxytocin neurons in the paraventricular nucleus drives cardiac sympathetic nerve activation following myocardial infarction in rats.Commun Biol. 2018 Oct 4;1:160. doi: 10.1038/s42003-018-0169-5. eCollection 2018. Commun Biol. 2018. PMID: 30320228 Free PMC article.
-
New medicines for spontaneous preterm birth prevention and preterm labour management: landscape analysis of the medicine development pipeline.BMC Pregnancy Childbirth. 2023 Jul 18;23(1):525. doi: 10.1186/s12884-023-05842-9. BMC Pregnancy Childbirth. 2023. PMID: 37464260 Free PMC article.
-
Tocolytics for delaying preterm birth: a network meta-analysis (0924).Cochrane Database Syst Rev. 2022 Aug 10;8(8):CD014978. doi: 10.1002/14651858.CD014978.pub2. Cochrane Database Syst Rev. 2022. PMID: 35947046 Free PMC article.
-
Structure and activity of conopressins: insights into in silico oxytocin/V2 receptor interactions, anti-inflammatory potential, and behavioural studies.RSC Med Chem. 2025 Jul 4. doi: 10.1039/d5md00288e. Online ahead of print. RSC Med Chem. 2025. PMID: 40726970 Free PMC article.
References
-
- American College of Obstetricians and Gynecologists , Committee on Practice Bulletins–Obstetrics . ACOG practice bulletin no. 127: management of preterm labor. Obstet Gynecol 2012; 119: 1308–1317. - PubMed
-
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371: 261–269. - PubMed
-
- Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr 2009; 154: 169–176. - PubMed
-
- Ananth CV, Friedman AM, Gyamfi‐Bannerman C. Epidemiology of moderate preterm, late preterm and early term delivery. Clin Perinatol 2013; 40: 601–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

