PINK1 regulates mitochondrial trafficking in dendrites of cortical neurons through mitochondrial PKA
- PMID: 28556983
- PMCID: PMC5554084
- DOI: 10.1111/jnc.14083
PINK1 regulates mitochondrial trafficking in dendrites of cortical neurons through mitochondrial PKA
Abstract
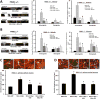
Mitochondrial Protein Kinase A (PKA) and PTEN-induced kinase 1 (PINK1), which is linked to Parkinson's disease, are two neuroprotective serine/threonine kinases that regulate dendrite remodeling and mitochondrial function. We have previously shown that PINK1 regulates dendrite morphology by enhancing PKA activity. Here, we show the molecular mechanisms by which PINK1 and PKA in the mitochondrion interact to regulate dendrite remodeling, mitochondrial morphology, content, and trafficking in dendrites. PINK1-deficient cortical neurons exhibit impaired mitochondrial trafficking, reduced mitochondrial content, fragmented mitochondria, and a reduction in dendrite outgrowth compared to wild-type neurons. Transient expression of wild-type, but not a PKA-binding-deficient mutant of the PKA-mitochondrial scaffold dual-specificity A Kinase Anchoring Protein 1 (D-AKAP1), restores mitochondrial trafficking, morphology, and content in dendrites of PINK1-deficient cortical neurons suggesting that recruiting PKA to the mitochondrion reverses mitochondrial pathology in dendrites induced by loss of PINK1. Mechanistically, full-length and cleaved forms of PINK1 increase the binding of the regulatory subunit β of PKA (PKA/RIIβ) to D-AKAP1 to enhance the autocatalytic-mediated phosphorylation of PKA/RIIβ and PKA activity. D-AKAP1/PKA governs mitochondrial trafficking in dendrites via the Miro-2/TRAK2 complex and by increasing the phosphorylation of Miro-2. Our study identifies a new role of D-AKAP1 in regulating mitochondrial trafficking through Miro-2, and supports a model in which PINK1 and mitochondrial PKA participate in a similar neuroprotective signaling pathway to maintain dendrite connectivity.
Keywords: Miro-2; PTEN-induced kinase 1; Parkinson's disease; Protein Kinase A; dual specificity A-kinase anchoring protein 1; mitochondrial trafficking.
© 2017 International Society for Neurochemistry.
Figures




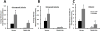

Similar articles
-
Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson's disease.Cell Death Differ. 2011 Dec;18(12):1914-23. doi: 10.1038/cdd.2011.74. Epub 2011 Jun 3. Cell Death Differ. 2011. PMID: 21637291 Free PMC article.
-
Beyond the mitochondrion: cytosolic PINK1 remodels dendrites through protein kinase A.J Neurochem. 2014 Mar;128(6):864-77. doi: 10.1111/jnc.12494. Epub 2013 Nov 13. J Neurochem. 2014. PMID: 24151868 Free PMC article.
-
Mitochondrial PKA Is Neuroprotective in a Cell Culture Model of Alzheimer's Disease.Mol Neurobiol. 2021 Jul;58(7):3071-3083. doi: 10.1007/s12035-021-02333-w. Epub 2021 Feb 23. Mol Neurobiol. 2021. PMID: 33624140 Free PMC article.
-
Role of protein kinase A in regulating mitochondrial function and neuronal development: implications to neurodegenerative diseases.Rev Neurosci. 2015;26(3):359-70. doi: 10.1515/revneuro-2014-0085. Rev Neurosci. 2015. PMID: 25741943 Free PMC article. Review.
-
Mechanisms of neurodegeneration in Parkinson's disease: keep neurons in the PINK1.Mech Ageing Dev. 2020 Jul;189:111277. doi: 10.1016/j.mad.2020.111277. Epub 2020 Jun 3. Mech Ageing Dev. 2020. PMID: 32504621 Review.
Cited by
-
Role of A-Kinase Anchoring Protein 1 in Retinal Ganglion Cells: Neurodegeneration and Neuroprotection.Cells. 2023 Jun 3;12(11):1539. doi: 10.3390/cells12111539. Cells. 2023. PMID: 37296658 Free PMC article. Review.
-
Kinase Signaling in Dendritic Development and Disease.Front Cell Neurosci. 2021 Feb 10;15:624648. doi: 10.3389/fncel.2021.624648. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33642997 Free PMC article. Review.
-
Neuronal autophagy and mitophagy in Parkinson's disease.Mol Aspects Med. 2021 Dec;82:100972. doi: 10.1016/j.mam.2021.100972. Epub 2021 Jun 12. Mol Aspects Med. 2021. PMID: 34130867 Free PMC article. Review.
-
Reduction of PINK1 or DJ-1 impair mitochondrial motility in neurites and alter ER-mitochondria contacts.J Cell Mol Med. 2018 Nov;22(11):5439-5449. doi: 10.1111/jcmm.13815. Epub 2018 Aug 22. J Cell Mol Med. 2018. PMID: 30133157 Free PMC article.
-
Brain-derived neurotrophic factor protects neurons by stimulating mitochondrial function through protein kinase A.J Neurochem. 2023 Oct;167(1):104-125. doi: 10.1111/jnc.15945. Epub 2023 Sep 9. J Neurochem. 2023. PMID: 37688457 Free PMC article.
References
-
- Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, Gottesman ME, Avvedimento EV, Feliciello A. Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. The Journal of biological chemistry. 2003;278:4286–4294. - PubMed
-
- Akabane S, Uno M, Tani N, Shimazaki S, Ebara N, Kato H, Kosako H, Oka T. PKA Regulates PINK1 Stability and Parkin Recruitment to Damaged Mitochondria through Phosphorylation of MIC60. Molecular cell. 2016;62:371–384. - PubMed
-
- Birsa N, Norkett R, Higgs N, Lopez-Domenech G, Kittler JT. Mitochondrial trafficking in neurons and the role of the Miro family of GTPase proteins. Biochem Soc Trans. 2013;41:1525–1531. - PubMed
-
- Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post‐translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

