Detection of gastric atrophy by circulating pepsinogens: A comparison of three assays
- PMID: 28557128
- PMCID: PMC5847288
- DOI: 10.1111/hel.12393
Detection of gastric atrophy by circulating pepsinogens: A comparison of three assays
Abstract
Background: Circulating levels of pepsinogens have been used in high gastric cancer-risk Asian and European populations to triage endoscopic evaluation for more severe pathology. There are different analytic methods with uncertain correlations. We therefore compared diagnostic performance of three commonly used pepsinogen assays to detect histologically confirmed gastric atrophy.
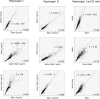
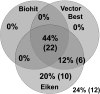
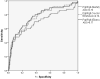
Methods: We tested plasma samples from adult patients with (n=50) and without (n=755) moderate or severe gastric corpus atrophy, as determined histologically by consensus of three expert pathologists. A single laboratory measured pepsinogens I (PgI) and II (PgII) using commercially available assays: two ELISA assays produced by Biohit (Finland) and Vector Best (Russia), and a latex agglutination assay from Eiken (Japan). Quantitative correlations were assessed by Spearman statistics. Receiver operating characteristic (ROC) curves vs histological diagnosis were calculated using both the manufacturers' and optimized cutoffs.
Results: Pepsinogen levels were highly correlated among the assays (pairwise Rhos: PgI≥0.84, PgII≥0.87; all P-values<.01). Based on manufacturers' cutoffs, sensitivities, specificities and areas under the ROC curve for detecting moderate to severe histological corpus atrophy by PgI/PgII were 44%/91%/0.70, 56%/84%/0.76, and 52%/90%/0.77 for Biohit, Vector Best and Eiken, respectively. Cutoffs optimized by ROC or data mining analyses did not substantially improve test performance.
Conclusions: Commercial assays for pepsinogen have good relative agreement but are imperfect tests for clinical diagnosis of gastric atrophy.
Impact: Pepsinogen testing alone does not provide sufficient information for gastric cancer risk stratification. Future investigations should focus on other potential markers, in combination with pepsinogens.
Keywords: atrophy; gastric cancer; pepsinogen; risk stratification; stomach.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures
References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer; 2013. (IARC CancerBase No. 11 [Internet]). Available from: http://globocan.iarc.fr, accessed on 03/12/2016.
-
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. - PubMed
-
- Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of gastroenterology and hepatology. 2009;24:1587–1600. - PubMed
- Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. - PMC - PubMed
-
- Dinis-Ribeiro M, Yamaki G, Miki K, Costa-Pereira A, Matsukawa M, Kurihara M. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen. 2004;11:141–147. - PubMed
-
- Samloff IM. Pepsinogens, pepsins, and pepsin inhibitors. Gastroenterology. 1971;60:586–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

