Extracellular vesicles do not contribute to higher circulating levels of soluble LRP1 in idiopathic dilated cardiomyopathy
- PMID: 28557183
- PMCID: PMC5661250
- DOI: 10.1111/jcmm.13211
Extracellular vesicles do not contribute to higher circulating levels of soluble LRP1 in idiopathic dilated cardiomyopathy
Abstract
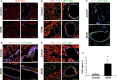
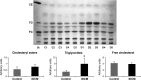
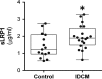
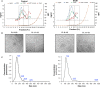
Idiopathic dilated cardiomyopathy (IDCM) is a frequent cause of heart transplantation. Potentially valuable blood markers are being sought, and low-density lipoprotein receptor-related protein 1 (LRP1) has been linked to the underlying molecular basis of the disease. This study compared circulating levels of soluble LRP1 (sLRP1) in IDCM patients and healthy controls and elucidated whether sLRP1 is exported out of the myocardium through extracellular vesicles (EVs) to gain a better understanding of the pathogenesis of the disease. LRP1 α chain expression was analysed in samples collected from the left ventricles of explanted hearts using immunohistochemistry. sLRP1 concentrations were determined in platelet-free plasma by enzyme-linked immunosorbent assay. Plasma-derived EVs were extracted by size-exclusion chromatography (SEC) and characterized by nanoparticle tracking analysis and cryo-transmission electron microscopy. The distributions of vesicular (CD9, CD81) and myocardial (caveolin-3) proteins and LRP1 α chain were assessed in SEC fractions by flow cytometry. LRP1 α chain was preferably localized to blood vessels in IDCM compared to control myocardium. Circulating sLRP1 was increased in IDCM patients. CD9- and CD81-positive fractions enriched with membrane vesicles with the expected size and morphology were isolated from both groups. The LRP1 α chain was not present in these SEC fractions, which were also positive for caveolin-3. The increase in circulating sLRP1 in IDCM patients may be clinically valuable. Although EVs do not contribute to higher sLRP1 levels in IDCM, a comprehensive analysis of EV content would provide further insights into the search for novel blood markers.
Keywords: biomarker; extracellular vesicles; idiopathic dilated cardiomyopathy; sLRP1; size-exclusion chromatography.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
Inverse relationship between raft LRP1 localization and non-raft ERK1,2/MMP9 activation in idiopathic dilated cardiomyopathy: potential impact in ventricular remodeling.Int J Cardiol. 2014 Oct 20;176(3):805-14. doi: 10.1016/j.ijcard.2014.07.270. Epub 2014 Aug 6. Int J Cardiol. 2014. PMID: 25131918
-
Circulating soluble low-density lipoprotein receptor-related protein 1 (sLRP1) concentration is associated with hypercholesterolemia: A new potential biomarker for atherosclerosis.Int J Cardiol. 2015 Dec 15;201:20-9. doi: 10.1016/j.ijcard.2015.07.085. Epub 2015 Aug 5. Int J Cardiol. 2015. PMID: 26285183
-
New insights into lipid raft function regulating myocardial vascularization competency in human idiopathic dilated cardiomyopathy.Atherosclerosis. 2013 Oct;230(2):354-64. doi: 10.1016/j.atherosclerosis.2013.08.009. Epub 2013 Aug 22. Atherosclerosis. 2013. PMID: 24075768
-
Umbilical cord blood-derived mesenchymal stem cells: new therapeutic weapons for idiopathic dilated cardiomyopathy?Int J Cardiol. 2014 Dec 20;177(3):809-18. doi: 10.1016/j.ijcard.2014.09.128. Epub 2014 Oct 2. Int J Cardiol. 2014. PMID: 25305679 Review.
-
Vascular dysfunction in idiopathic dilated cardiomyopathy.Nat Rev Cardiol. 2009 Sep;6(9):590-8. doi: 10.1038/nrcardio.2009.130. Epub 2009 Jul 28. Nat Rev Cardiol. 2009. PMID: 19636323 Review.
Cited by
-
Soluble LRP1 is an independent biomarker of epicardial fat volume in patients with type 1 diabetes mellitus.Sci Rep. 2018 Jan 18;8(1):1054. doi: 10.1038/s41598-018-19230-3. Sci Rep. 2018. PMID: 29348672 Free PMC article.
-
Identification of new biophysical markers for pathological ventricular remodelling in tachycardia-induced dilated cardiomyopathy.J Cell Mol Med. 2018 Sep;22(9):4197-4208. doi: 10.1111/jcmm.13699. Epub 2018 Jun 19. J Cell Mol Med. 2018. PMID: 29921039 Free PMC article.
-
Midkine's Role in Cardiac Pathology.J Cardiovasc Dev Dis. 2017 Sep;4(3):13. doi: 10.3390/jcdd4030013. Epub 2017 Aug 24. J Cardiovasc Dev Dis. 2017. PMID: 28920060 Free PMC article.
-
Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography.Cell Mol Life Sci. 2019 Jun;76(12):2369-2382. doi: 10.1007/s00018-019-03071-y. Epub 2019 Mar 19. Cell Mol Life Sci. 2019. PMID: 30891621 Free PMC article. Review.
References
-
- Towbin JA, Bowles NE. The failing heart. Nature. 2002; 415: 227–33. - PubMed
-
- Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010; 375: 752–62. - PubMed
-
- Towbin JA, Lorts A. Arrhythmias and dilated cardiomyopathy common pathogenetic pathways? J Am Coll Cardiol. 2011; 57: 2169–71. - PubMed
-
- Roura S, Planas F, Prat‐Vidal C, et al Idiopathic dilated cardiomyopathy exhibits defective vascularization and vessel formation. Eur J Heart Fail. 2007; 9: 995–1002. - PubMed
-
- Roura S, Bayes‐Genis A. Vascular dysfunction in idiopathic dilated cardiomyopathy. Nat Rev Cardiol. 2009; 6: 590–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

