Amino acid metabolites that regulate G protein signaling during osmotic stress
- PMID: 28558063
- PMCID: PMC5469498
- DOI: 10.1371/journal.pgen.1006829
Amino acid metabolites that regulate G protein signaling during osmotic stress
Abstract
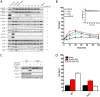
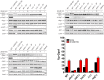
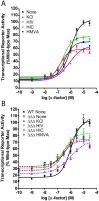
All cells respond to osmotic stress by implementing molecular signaling events to protect the organism. Failure to properly adapt can lead to pathologies such as hypertension and ischemia-reperfusion injury. Mitogen-activated protein kinases (MAPKs) are activated in response to osmotic stress, as well as by signals acting through G protein-coupled receptors (GPCRs). For proper adaptation, the action of these kinases must be coordinated. To identify second messengers of stress adaptation, we conducted a mass spectrometry-based global metabolomics profiling analysis, quantifying nearly 300 metabolites in the yeast S. cerevisiae. We show that three branched-chain amino acid (BCAA) metabolites increase in response to osmotic stress and require the MAPK Hog1. Ectopic addition of these BCAA derivatives promotes phosphorylation of the G protein α subunit and dampens G protein-dependent transcription, similar to that seen in response to osmotic stress. Conversely, genetic ablation of Hog1 activity or the BCAA-regulatory enzymes leads to diminished phosphorylation of Gα and increased transcription. Taken together, our results define a new class of candidate second messengers that mediate cross talk between osmotic stress and GPCR signaling pathways.
Conflict of interest statement
EMK was previously a paid employee of Metabolon, a for-profit company. After completing the research she relocated to Attain LLC, which had no role in the project. RPM is currently a paid employee of Metabolon, a for-profit company. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Metabolon provided no employment or consultancy to the other authors, and have claimed no rights to possible patents or products that may arise from the research.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

