Collision-induced dissociation of phenethylamides: role of ion-neutral complexes
- PMID: 28558170
- PMCID: PMC5555735
- DOI: 10.1002/rcm.7915
Collision-induced dissociation of phenethylamides: role of ion-neutral complexes
Abstract
Rationale: Phenethylamides are a large group of naturally occurring molecules found both in the plant and animal kingdoms. In addition, they are used as intermediates for the synthesis of pharmaceutically important dihydro- and tetrahydroisoquinolines. To enable efficient characterization of this class of molecules, a detailed mass spectrometric fragmentation study of a broad series of analogs was carried out.
Methods: The test compounds were synthesized using standard methods for amide bond formation. Low-energy high-resolution tandem mass spectra were acquired on a hybrid quadrupole/time-of-flight mass spectrometer using positive ion electrospray ionization.
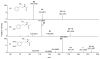
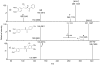
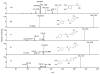
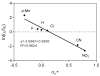
Results: A total of 26 analogs were investigated in the study. Fragmentation of phenethylamides was found to proceed via intermediate ion-neutral complexes. The complexes can break down via multiple pathways including dissociation, proton transfer, Friedel-Crafts acylation, and single electron transfer. The relative contribution of each of these pathways strongly depends on the structure of the coupling amine and acid.
Conclusions: A general scheme for the fragmentation of phenethylamides was developed. This study further extends the knowledge base of the ion-neutral complex by discovering Friedel-Crafts acylation as a novel reaction. The strong influence of minor structural modifications on the fragmentation patterns highlights the importance of testing many analogs in order to fully predict a fragmentation pattern of a particular class of molecules.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures





Similar articles
-
Fragmentation reactions of N-benzyltetrahydroquinolines in electrospray ionization mass spectrometry: the roles of ion/neutral complex intermediates.Rapid Commun Mass Spectrom. 2014 Jun 30;28(12):1381-6. doi: 10.1002/rcm.6918. Rapid Commun Mass Spectrom. 2014. PMID: 24797950
-
Identifying the proton transfer reaction mechanism via a proton-bound dimeric intermediate for esomeprazoles by a kinetic method combined with density functional theory calculations.Rapid Commun Mass Spectrom. 2014 May 15;28(9):1045-50. doi: 10.1002/rcm.6877. Rapid Commun Mass Spectrom. 2014. PMID: 24677526
-
Characterization of complex, heterogeneous lipid A samples using HPLC-MS/MS technique III. Positive-ion mode tandem mass spectrometry to reveal phosphorylation and acylation patterns of lipid A.J Mass Spectrom. 2018 Feb;53(2):146-161. doi: 10.1002/jms.4046. J Mass Spectrom. 2018. PMID: 29144587
-
Ion activation methods for tandem mass spectrometry.J Mass Spectrom. 2004 Oct;39(10):1091-112. doi: 10.1002/jms.703. J Mass Spectrom. 2004. PMID: 15481084 Review.
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
Cited by
-
Structure Annotation of All Mass Spectra in Untargeted Metabolomics.Anal Chem. 2019 Feb 5;91(3):2155-2162. doi: 10.1021/acs.analchem.8b04698. Epub 2019 Jan 16. Anal Chem. 2019. PMID: 30608141 Free PMC article.
-
Atmospheric Pressure Chemical Ionization Q-Orbitrap Mass Spectrometry Analysis of Gas-Phase High-Energy Dissociation Routes of Triarylamine Derivatives.Molecules. 2024 Dec 9;29(23):5807. doi: 10.3390/molecules29235807. Molecules. 2024. PMID: 39683964 Free PMC article.
References
-
- Boonen J, Bronselaer A, Nielandt J, Veryser L, De Tre G, De Spiegeleer B. Alkamid database: Chemistry, occurrence and functionality of plant N-alkylamides. J Ethnopharmacol. 2012;142:563. - PubMed
-
- Rios M. Drug Discovery Research in Pharmacognosy. InTech; Rijeka: 2012. p. 107. www.intechopen.com.
-
- Di Antonio M, Doria F, Richter SN, Bertipaglia C, Mella M, Sissi C, Palumbo M, Freccero M. Quinone Methides Tethered to Naphthalene Diimides as Selective G-Quadruplex Alkylating Agents. J Am Chem Soc. 2009;131:13132. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
