Global Kinetic Mechanism of Microsomal Glutathione Transferase 1 and Insights into Dynamic Enzyme Activation
- PMID: 28558199
- PMCID: PMC5954419
- DOI: 10.1021/acs.biochem.7b00285
Global Kinetic Mechanism of Microsomal Glutathione Transferase 1 and Insights into Dynamic Enzyme Activation
Abstract
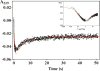
Microsomal glutathione transferase 1 (MGST1) has a unique ability to be activated, ≤30-fold, by modification with sulfhydryl reagents. MGST1 exhibits one-third-of-the-sites reactivity toward glutathione and hence heterogeneous binding to different active sites in the homotrimer. Limited turnover stopped-flow kinetic measurements of the activated enzyme allowed us to more accurately determine the KD for the "third" low-affinity GSH binding site (1.4 ± 0.3 mM). The rate of thiolate formation, k2 (0.77 ± 0.06 s-1), relevant to turnover, could also be determined. By deriving the steady-state rate equation for a random sequential mechanism for MGST1, we can predict KM, kcat, and kcat/KM values from these and previously determined pre-steady-state rate constants (all determined at 5 °C). To assess whether the pre-steady-state behavior can account for the steady-state kinetic behavior, we have determined experimental values for kinetic parameters at 5 °C. For reactive substrates and the activated enzyme, data for the microscopic steps account for the global mechanism of MGST1. For the unactivated enzyme and more reactive electrophilic substrates, pre-steady-state and steady-state data can be reconciled only if a more active subpopulation of MGST1 is assumed. We suggest that unactivated MGST1 can be partially activated in its unmodified form. The existence of an activated subpopulation (approximately 10%) could be demonstrated in limited turnover experiments. We therefore suggest that MSGT1 displays a preexisting dynamic equilibrium between high- and low-activity forms.
Conflict of interest statement
Richard N. Armstrong sadly passed in 2015. The authors declare no competing financial interests.
Figures









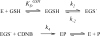

Similar articles
-
Kinetic analysis of the slow ionization of glutathione by microsomal glutathione transferase MGST1.Biochemistry. 2001 Mar 20;40(11):3378-84. doi: 10.1021/bi0023394. Biochemistry. 2001. PMID: 11258959
-
Kinetic characterization of thiolate anion formation and chemical catalysis of activated microsomal glutathione transferase 1.Biochemistry. 2004 Jul 13;43(27):8869-77. doi: 10.1021/bi0492511. Biochemistry. 2004. PMID: 15236595
-
Enzymology of reactive intermediate protection: kinetic analysis and temperature dependence of the mesophilic membrane protein catalyst MGST1.FEBS J. 2023 Jul;290(13):3448-3460. doi: 10.1111/febs.16754. Epub 2023 Mar 14. FEBS J. 2023. PMID: 36808476
-
Microsomal glutathione transferase 1 in cancer and the regulation of ferroptosis.Adv Cancer Res. 2023;160:107-132. doi: 10.1016/bs.acr.2023.05.001. Epub 2023 Jul 21. Adv Cancer Res. 2023. PMID: 37704286 Free PMC article. Review.
-
Microsomal glutathione transferase 1: mechanism and functional roles.Drug Metab Rev. 2011 May;43(2):300-6. doi: 10.3109/03602532.2011.558511. Drug Metab Rev. 2011. PMID: 21495795 Review.
References
-
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. - PubMed
-
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. - PubMed
-
- Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. - PubMed
-
- Johansson K, Jarvliden J, Gogvadze V, Morgenstern R. Multiple roles of microsomal glutathione transferase 1 in cellular protection: a mechanistic study. Free Radic Biol Med. 2010;49:1638–1645. - PubMed
-
- Park HS, Nam SH, Lee JK, Yoon CN, Mannervik B, Benkovic SJ, Kim HS. Design and evolution of new catalytic activity with an existing protein scaffold. Science. 2006;311:535–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

