Role of human rhinovirus in triggering human airway epithelial-mesenchymal transition
- PMID: 28558698
- PMCID: PMC5450126
- DOI: 10.1186/s12931-017-0595-9
Role of human rhinovirus in triggering human airway epithelial-mesenchymal transition
Abstract
Background: Structural changes in the airways, collectively referred to as airway remodeling, are a characteristic feature of asthma, and are now known to begin in early life. Human rhinovirus (HRV)-induced wheezing illnesses during early life are a potential inciting stimulus for remodeling. Increased deposition of matrix proteins causes thickening of the lamina reticularis, which is a well-recognized component of airway remodeling. Increased matrix protein deposition is believed to be due to the presence of increased numbers of activated mesenchymal cells (fibroblasts/myofibroblasts) in the subepithelial region of asthmatic airways. The origin of these increased mesenchymal cells is not clear, but one potential contributor is the process of epithelial-mesenchymal transition (EMT). We hypothesized that HRV infection may help to induce EMT.
Methods: We used the BEAS-2B human bronchial epithelial cells line, which uniformly expresses the major group HRV receptor, to examine the effects of stimulation with HRV alone, transforming growth factor-β1 (TGF-β1), alone, and the combination, on induction of changes consistent with EMT. Western blotting was used to examine expression of epithelial and mesenchymal phenotypic marker proteins and selected signaling molecules. Cell morphology was also examined.
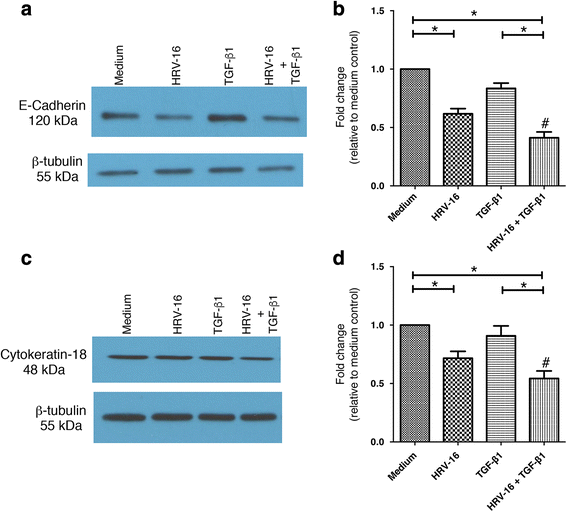
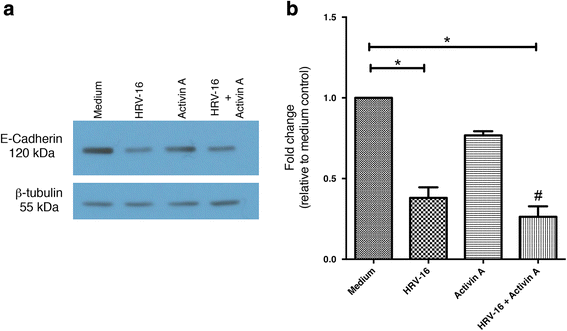
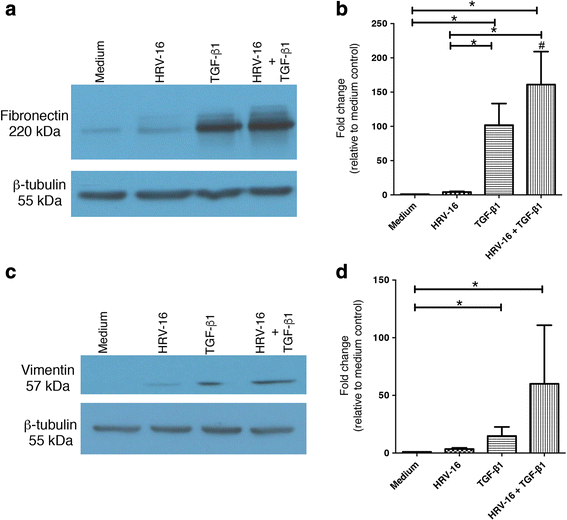
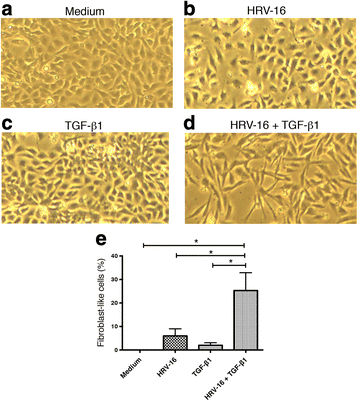
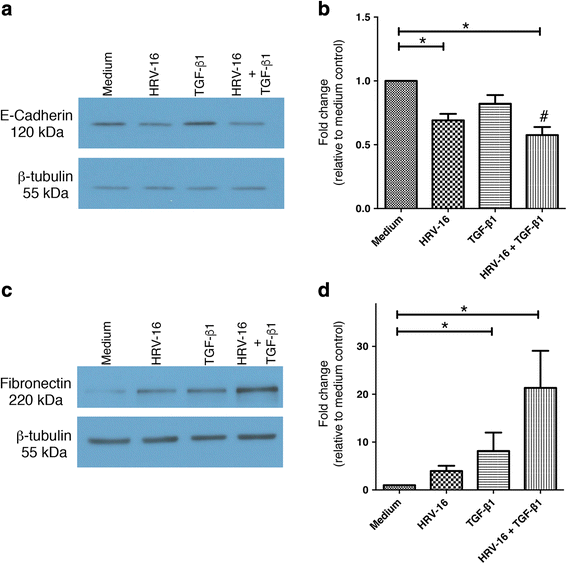
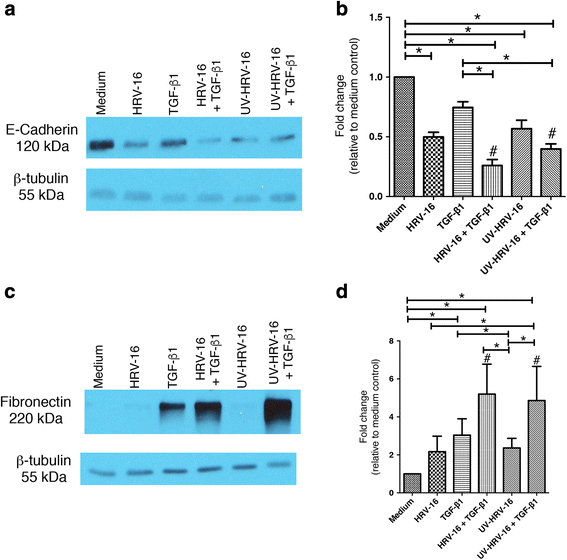
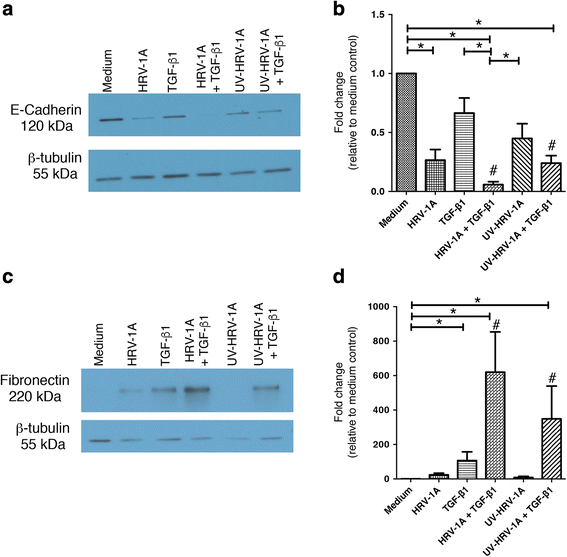
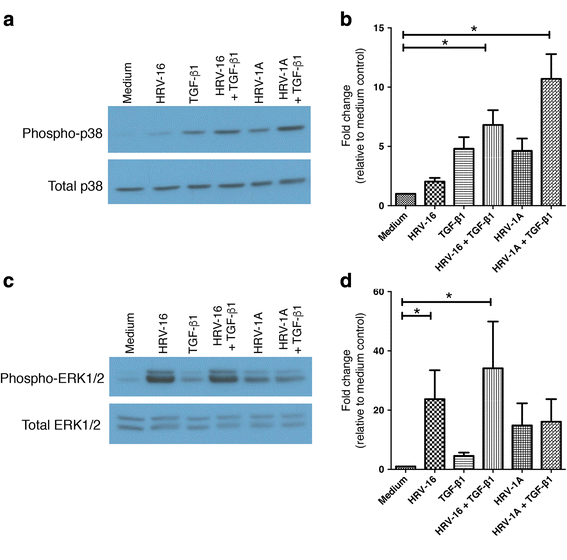
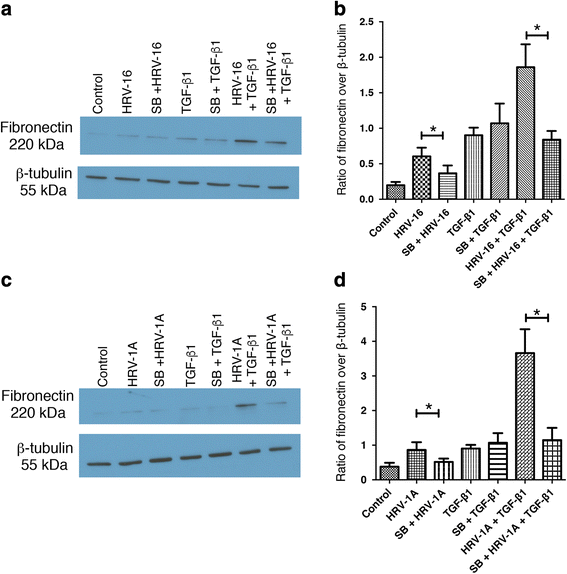
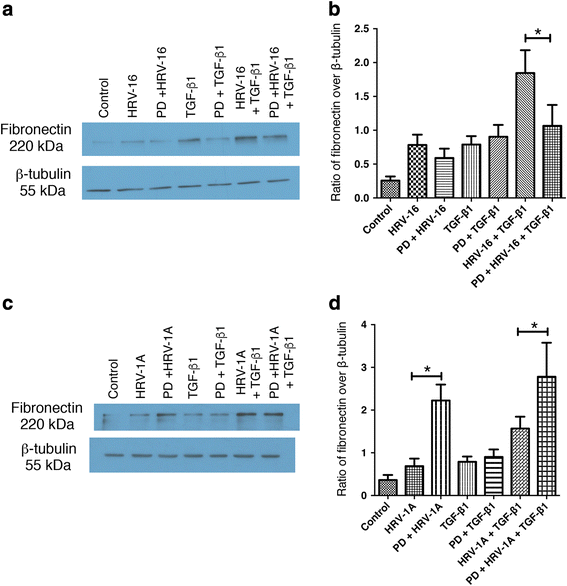
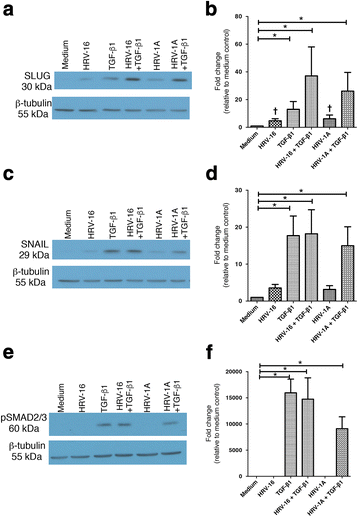
Results: In this study, we show that two different strains of HRV, which use two different cellular receptors, are each capable of triggering phenotypic changes consistent with EMT. Moreover, both HRV serotypes synergistically induced changes consistent with EMT when used in the presence of TGF-β1. Morphological changes were also most pronounced with the combination of HRV and TGF-β1. Viral replication was not essential for phenotypic changes. The synergistic interactions between HRV and TGF-β1 were mediated, at least in part, via activation of mitogen activated protein kinase pathways, and via induction of the transcription factor SLUG.
Conclusions: These data support a role for HRV in the induction of EMT, which may contribute to matrix protein deposition and thickening of the lamina reticularis in airways of patients with asthma.
Keywords: E-cadherin; Epithelial-mesenchymal transition; Fibronectin; Human rhinovirus; MAP kinases; SLUG; Transforming growth factor-β1.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

