Electronic applications for the CFQ-R scoring
- PMID: 28558706
- PMCID: PMC5450183
- DOI: 10.1186/s12931-017-0592-z
Electronic applications for the CFQ-R scoring
Abstract
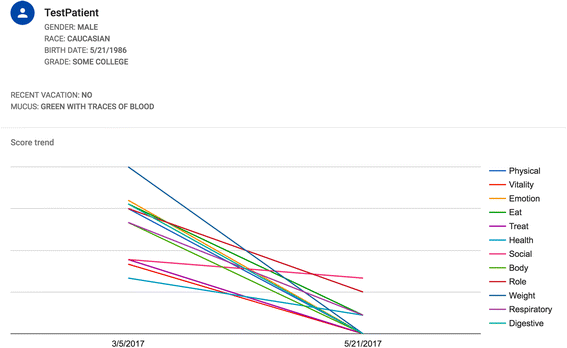
Patient reported outcomes (PROs) have become widely accepted outcome measures in cystic fibrosis (CF) and other respiratory diseases. The Cystic Fibrosis-Questionnaire-Revised (CFQ-R) is the best validated and most widely used PRO for CF. Data collection can be time-intensive, and electronic platforms would greatly facilitate the feasibility, utility and accuracy of administration of the CFQ-R. Given that the CFQ-R is utilized in virtually all clinical trials worldwide and is increasingly integrated into clinical practice, we developed a software application that will help users to administer, score and save CFQ-R data for all versions. All codes are open access, which will enable other PRO users to design similar applications for other respiratory diseases, such as primary ciliary dyskinesia and non-CF bronchiectasis.
Keywords: Application; Cystic fibrosis; Patient reported outcomes; Software.
Figures
References
-
- U.S. Food and Drug Administration . Guidance for industry patientreported outcome measures: Use in medical product development to support labeling claims. 2009. - PubMed
-
- Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, Hess R, Miller DM, Reeve DM, Santana M. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21:1305–14. doi: 10.1007/s11136-011-0054-x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

