FGF2/FGFR1 regulates autophagy in FGFR1-amplified non-small cell lung cancer cells
- PMID: 28558758
- PMCID: PMC5450166
- DOI: 10.1186/s13046-017-0534-0
FGF2/FGFR1 regulates autophagy in FGFR1-amplified non-small cell lung cancer cells
Abstract
Background: Autophagy is a conserved catabolic process to degrade cellular organelles. The role of autophagy in cancer development is complex. Amplification of fibroblast growth factor receptor 1 (FGFR1) is one of the most frequent targets in lung squamous cell carcinoma (SQCC). Whether fibroblast growth factor 2 (FGF2)/FGFR1 contributes to the regulation of autophagy remains elusive.
Methods: Autophagic activity was evaluated by immunoblotting for microtubule-associated protein 1 light chain 3 (LC3), formation of GFP-LC3 puncta, and monodansylcadaverine (MDC) staining. The effect of autophagy inhibition on cell survival was assessed by cell viability and apoptosis assays.
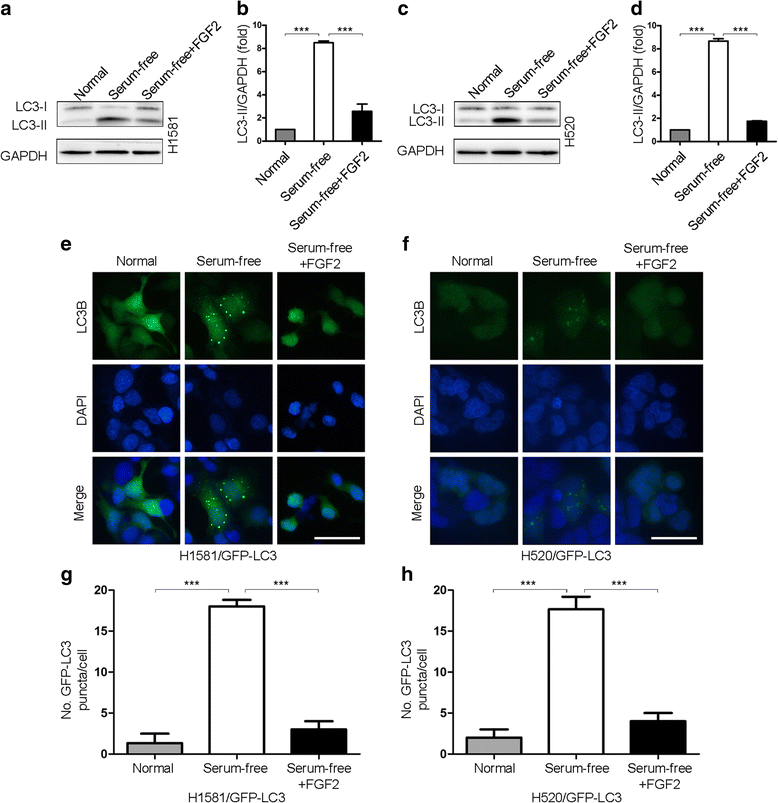
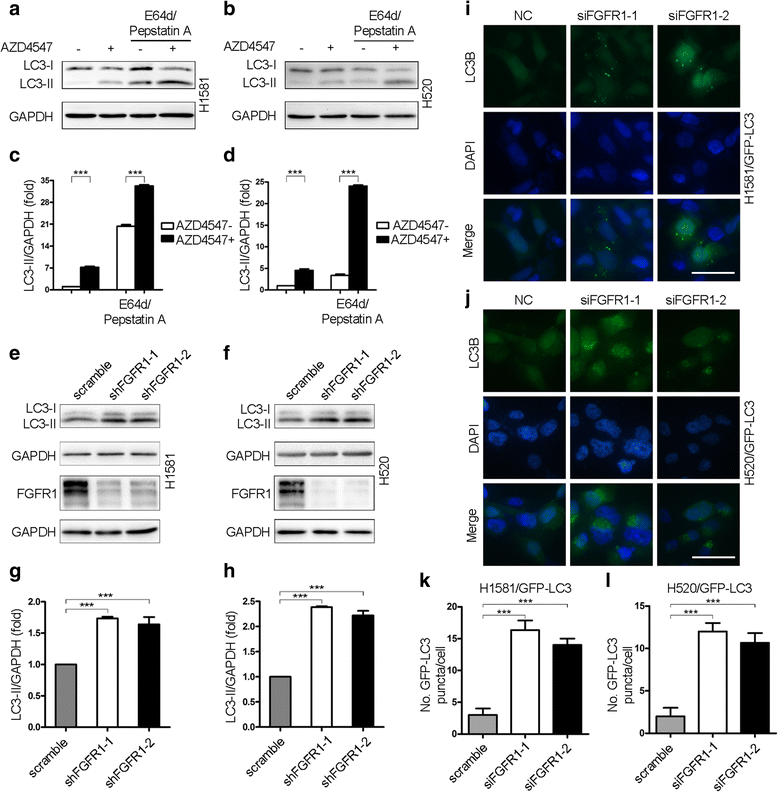
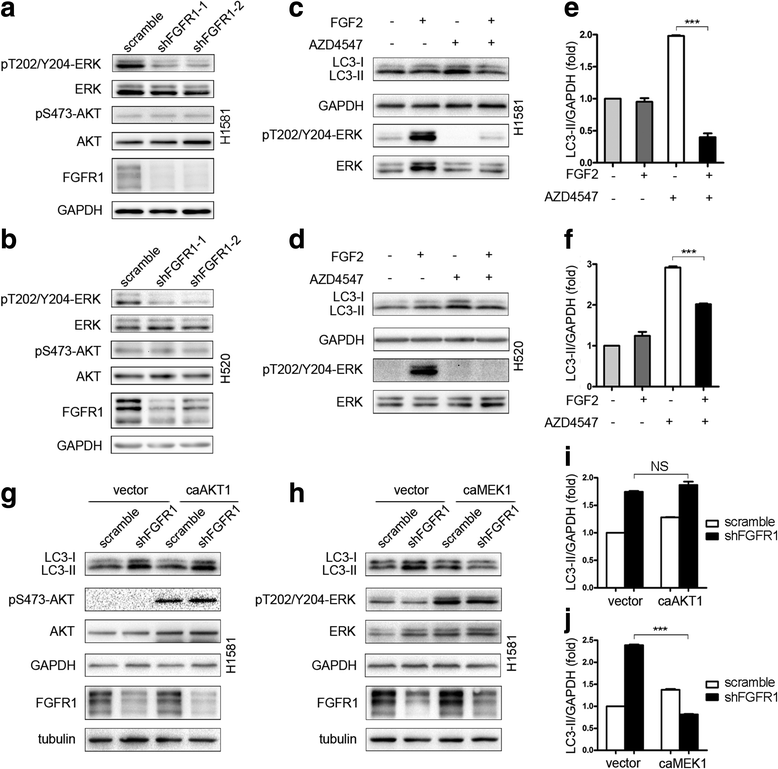
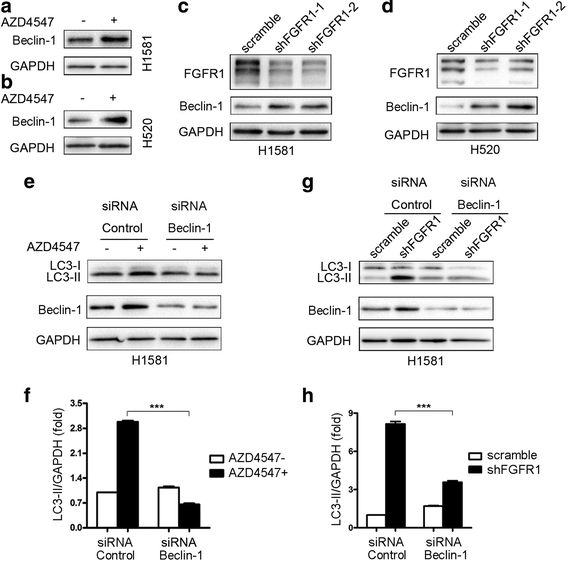
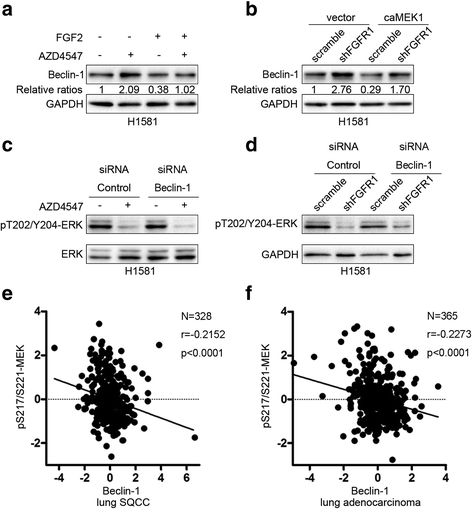
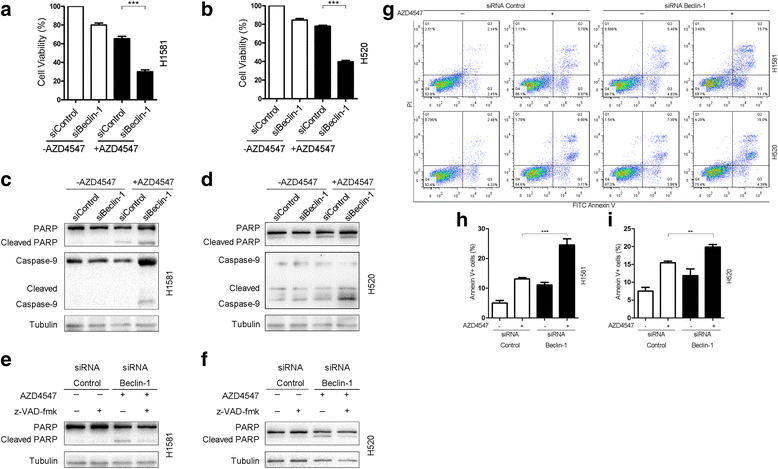
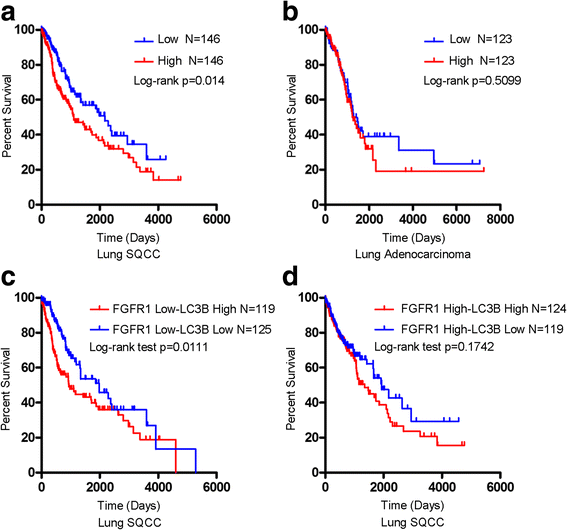
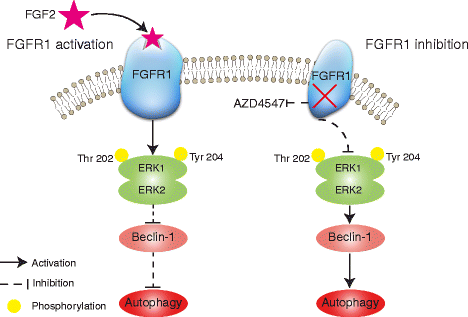
Results: We elucidated that FGFR1 activation suppressed autophagy. Pharmacological or genetic inhibition of FGFR1 by AZD4547 or FGFR1 short hairpin RNA (shRNA) induced autophagy in FGFR1-amplified non-small cell lung cancer (NSCLC) cells, H1581 and H520 cells. Mechanistic study revealed that the induction of autophagy by FGFR1 inhibition was mediated through inhibiting the ERK/MAPK pathway not by AKT pathway, accompanied by upregulation of beclin-1. Furthermore, activation of ERK/MAPK by transfection with a constitutively active MEK1 (caMEK1) construct or knockdown of beclin-1 by RNAi could attenuate autophagy induced by FGFR1 inhibition. Beclin-1 expression was inversely correlated with MEK1 phosphorylation. Inhibition of autophagy by beclin-1 silencing could enhance apoptosis after AZD4547 treatment in H1581 and H520 cells. High levels of LC3B mRNA was a marker of poor prognosis in NSCLC patients.
Conclusions: Simultaneously inhibiting FGFR1 and autophagy could enhance cell death which should be further explored in vivo.
Keywords: Autophagy; Beclin-1; ERK; FGFR1; NSCLC.
Figures
Similar articles
-
FGFR1 promotes the stem cell-like phenotype of FGFR1-amplified non-small cell lung cancer cells through the Hedgehog pathway.Oncotarget. 2016 Mar 22;7(12):15118-34. doi: 10.18632/oncotarget.7701. Oncotarget. 2016. PMID: 26936993 Free PMC article.
-
Translating the therapeutic potential of AZD4547 in FGFR1-amplified non-small cell lung cancer through the use of patient-derived tumor xenograft models.Clin Cancer Res. 2012 Dec 15;18(24):6658-67. doi: 10.1158/1078-0432.CCR-12-2694. Epub 2012 Oct 18. Clin Cancer Res. 2012. PMID: 23082000
-
Resistance mediated by alternative receptor tyrosine kinases in FGFR1-amplified lung cancer.Carcinogenesis. 2017 Oct 26;38(11):1063-1072. doi: 10.1093/carcin/bgx091. Carcinogenesis. 2017. PMID: 28968756
-
Fibroblast Growth Factor Receptor 1 Gene Amplification in Nonsmall Cell Lung Cancer.Chin Med J (Engl). 2016 Dec 5;129(23):2868-2872. doi: 10.4103/0366-6999.194649. Chin Med J (Engl). 2016. PMID: 27901003 Free PMC article. Review.
-
Upregulation of Beclin 1 in the ischemic penumbra.Autophagy. 2008 Feb;4(2):227-9. doi: 10.4161/auto.5339. Epub 2007 Nov 27. Autophagy. 2008. PMID: 18075295 Review.
Cited by
-
Interplay of autophagy and cancer stem cells in hepatocellular carcinoma.Mol Biol Rep. 2021 Apr;48(4):3695-3717. doi: 10.1007/s11033-021-06334-9. Epub 2021 Apr 24. Mol Biol Rep. 2021. PMID: 33893928 Review.
-
Bioinformatics analysis of miRNAs germacrone protection on diabetic nephropathy.Sci Rep. 2024 Dec 30;14(1):31754. doi: 10.1038/s41598-024-81944-4. Sci Rep. 2024. PMID: 39738220 Free PMC article.
-
E3 ligase MIB1 regulates STAT1/P21 signaling via regulation of FGFR1 in colorectal cancer.Genes Genomics. 2025 Jun;47(6):663-670. doi: 10.1007/s13258-025-01629-8. Epub 2025 Mar 10. Genes Genomics. 2025. PMID: 40063182
-
IEO model: A novel concept describing the complete metastatic process in the liver microenvironment.Oncol Lett. 2020 Jun;19(6):3627-3633. doi: 10.3892/ol.2020.11525. Epub 2020 Apr 10. Oncol Lett. 2020. PMID: 32391088 Free PMC article. Review.
-
FGF19 promotes cell autophagy and cisplatin chemoresistance by activating MAPK signaling in ovarian cancer.PeerJ. 2023 Feb 2;11:e14827. doi: 10.7717/peerj.14827. eCollection 2023. PeerJ. 2023. PMID: 36751636 Free PMC article.
References
-
- Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3:264–279. doi: 10.1158/2159-8290.CD-12-0362. - DOI - PubMed
-
- Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, Ullrich RT, Menon R, Maier S, Soltermann A, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

