In vitro quality evaluation of leading brands of ciprofloxacin tablets available in Bangladesh
- PMID: 28558832
- PMCID: PMC5450125
- DOI: 10.1186/s13104-017-2507-y
In vitro quality evaluation of leading brands of ciprofloxacin tablets available in Bangladesh
Abstract
Background: Ciprofloxacin is a broad-spectrum antibiotic that acts against a number of bacterial infections. The study was carried out to examine the in vitro quality control tests for ten leading brands of ciprofloxacin hydrochloride 500 mg tablet formulation, registered in Bangladesh by Directorate General of Drug Administration. The quality control parameters of ten different brands of ciprofloxacin hydrochloride 500 mg tablets were determined by weight variation, friability, hardness, disintegration, dissolution and assay tests. All the tablets were evaluated for conformity with United States Pharmacopoeia-National Formulary (USP-NF) and British Pharmacopoeia (BP) standards.
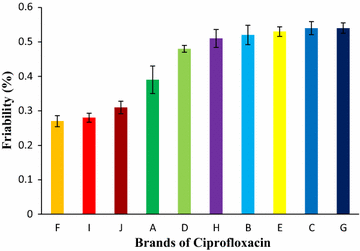
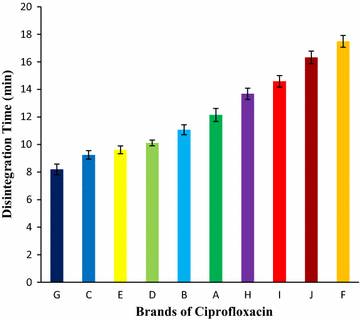
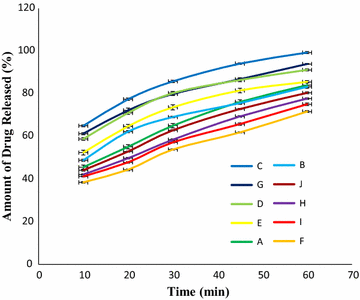
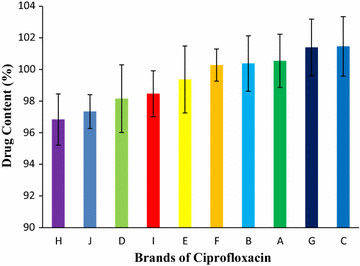
Results: Among ten brands of tablets Brand C had lower mean weight variation of 1.59% and Brand E had highest mean weight variation of 3.32%. For friability test Brand F had lowest mean friability (0.27%) and Brand G had highest mean friability (0.54%). Among ten brands mean lowest and highest hardness were founded in Brand G (4.49 kg/cm2) and Brand F (7.13 kg/cm2) respectively. The disintegration time for ten brands of ciprofloxacin tablet obtained were in the subsequent order: Brand G (8.19 min) < Brand C (9.25 min) < Brand E (9.61 min) < Brand D (10.11 min) < Brand B (11.07 min) < Brand A (12.15 min) < Brand H (13.68 min) < Brand I (14.59 min) < Brand J (16.32 min) < Brand F (17.49 min). Among ten brands for dissolution test mean percentages of drug release were not less than 80% in 45 min for four tablets (Brand E, 81.52%; Brand D, 86.44%; Brand G, 86.82% and Brand C, 94.12%), consequently they met BP standard and as per USP-NF standard six brands (Brand B, 75.62%; Brand A, 76.18%; Brand E, 81.52%; Brand D, 86.44%; Brand G, 86.82% and Brand C, 94.12%) had released not less than 75% drugs, so they also complied with the standard. The percentages of the drug content of the ten brands of ciprofloxacin tablet were obtained in the following sequence: Brand H (96.84%) < Brand J (97.34%) < Brand D (98.15%) < Brand I (98.47%) < Brand E (99.37%) < Brand F (100.28%) < Brand B (100.38%) < Brand A (100.54%) < Brand G (101.39%) < Brand C (101.46%). All of the brands met the BP and USP-NF specifications for assay. First-order, Higuchi and Korsmeyer-Peppas kinetics model fit for all of the mentioned ten brands.
Conclusion: The present study revealed that all of the leading brands of this tablet met the quality control parameters as per pharmacopoeial specifications except dissolution test for four brands (Brand J, Brand H, Brand I, and Brand F).
Keywords: Ciprofloxacin hydrochloride; Leading brands; Pharmacopoeial specifications; Quality control.
Figures



References
-
- Hamam HAB. Comparative in vitro evaluation of generic ciprofloxacin hydrochloride tablets. World J Pharm Pharm Sci. 2014;3(12):388–390.
-
- Hailu GS, Gutema GB, Asefaw AA, Hussen DA, Hadera MG. Comparative assessment of the physicochemical and in vitro bioavailability equivalence of cotrimoxazole tablets marketed in Tigray, Ethiopia. Int J Pharm Sci Res. 2011;2(12):3210–3218.
-
- Anonymous. 19th WHO model list of essential medicines. http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-M.... Accessed 12 Oct 2015.
-
- Anonymous. Ciprofloxacin hydrochloride. http://www.drugs.com/monograph/ciprofloxacin-hydrochloride.html. Accessed 12 Oct 2015.
-
- Hamilton Richard J. Tarascon pharmacopoeia. 15. Burlington: Jones & Bartlett Publishers; 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

