Reduction in clinically important deterioration in chronic obstructive pulmonary disease with aclidinium/formoterol
- PMID: 28558833
- PMCID: PMC5450266
- DOI: 10.1186/s12931-017-0583-0
Reduction in clinically important deterioration in chronic obstructive pulmonary disease with aclidinium/formoterol
Abstract
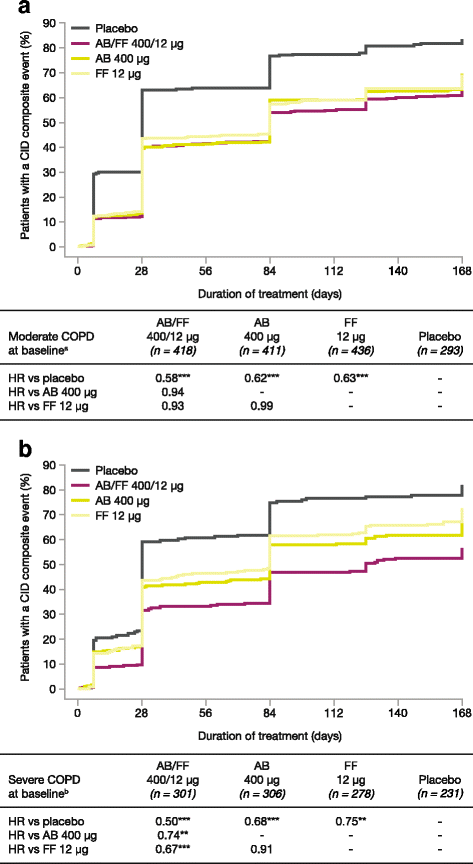
Background: 'Clinically important deterioration' (CID) is a composite endpoint measuring worsening of the key clinical features of chronic obstructive pulmonary disease (COPD), namely lung function, patient-reported outcomes, and exacerbations. ACLIFORM and AUGMENT were two 24-week, randomized, double-blind, phase III studies assessing twice-daily (BID) aclidinium bromide (AB) 400 μg/formoterol fumarate (FF) 12 μg. This pooled post-hoc analysis assessed the effects of AB/FF 400/12 μg on both first and sustained CID events versus placebo and monotherapies in patients with moderate to severe COPD.
Methods: A first CID event was defined as the occurrence of a moderate/severe exacerbation or the worsening from baseline in ≥1 of the following: trough forced expiratory volume in 1 second (FEV1; ≥100 mL), Transition Dyspnea Index (TDI) focal score (≥1 unit), or St George's Respiratory Questionnaire (SGRQ) total score (≥4 units). A 'sustained' CID was defined as a worsening maintained at all subsequent visits from appearance to week 24 or a moderate/severe exacerbation at any time. CID events were assessed at three visits (weeks 4, 12, and 24); trough FEV1 was also measured at weeks 1 and 18.
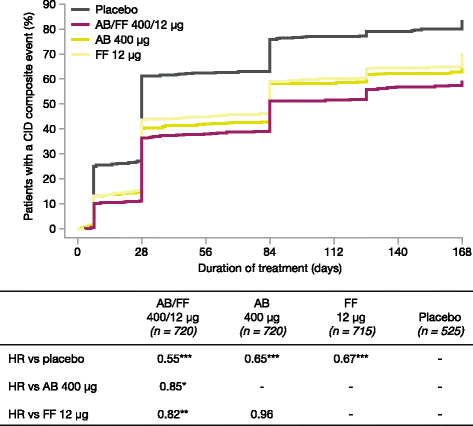
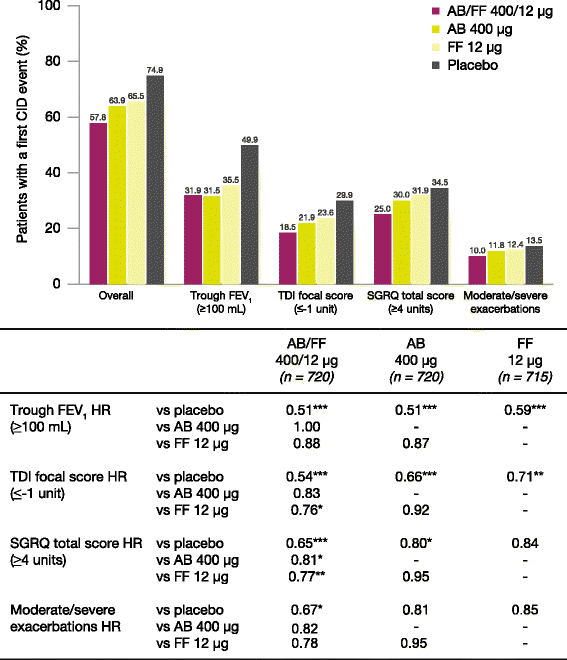
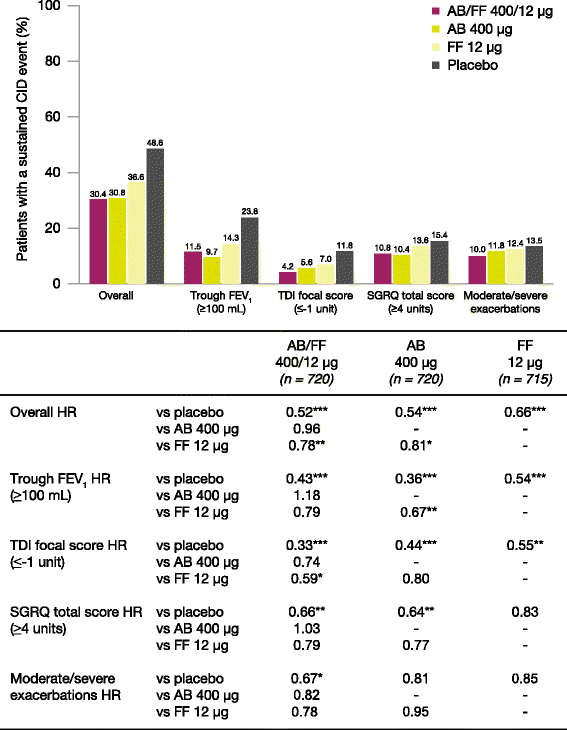
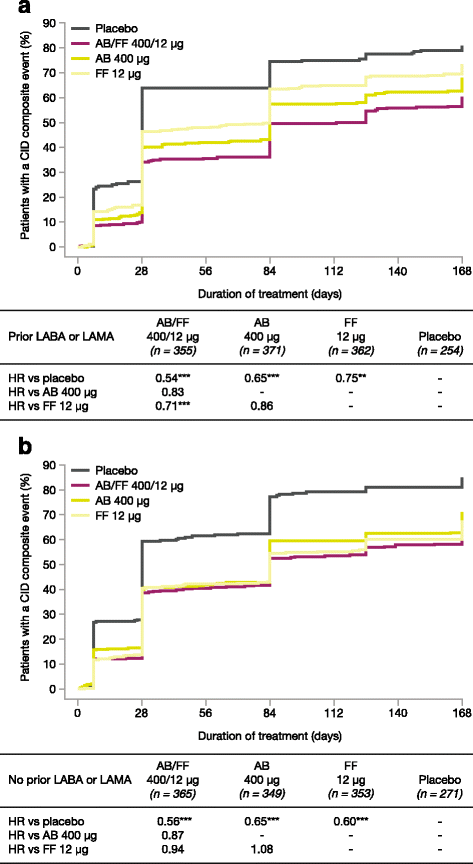
Results: AB/FF 400/12 μg reduced the risk of a first CID event by 45% versus placebo (hazard ratio [HR] 0.55, p < 0.001), 18% versus FF 12 μg (HR 0.82, p < 0.01), and 15% versus AB 400 μg (HR 0.85, p < 0.05). Similarly, AB/FF 400/12 μg reduced the risk of a sustained CID event by 48% versus placebo (HR 0.52, p < 0.001) and 22% versus FF 12 μg (HR 0.78, p < 0.01). AB/FF 400/12 μg reduced the risk of a first or sustained CID event for all four components versus placebo (trough FEV1 and TDI, first and sustained CID, all p < 0.001; SGRQ first CID p < 0.001; SGRQ sustained CID, p < 0.01; exacerbations first and sustained CID, both p < 0.05) and TDI and SGRQ versus FF 12 μg (TDI, first and sustained CID both p < 0.05; SGRQ first CID p < 0.01), and SGRQ versus AB 400 μg (first CID, p < 0.05).
Conclusions: AB/FF 400/12 μg BID may provide greater airway stability and fewer exacerbations or deteriorations in lung function, health status, or dyspnea compared with placebo or monotherapies.
Trial registration: Clinicaltrials.gov NCT01462942 (ACLIFORM); registered 26 October 2011. Clinicaltrials.gov NCT01437397 (AUGMENT); registered 19 September 2011.
Keywords: Bronchodilation; COPD; Chronic respiratory disease; LABA; LAMA.
Figures
References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. 2017;49:1700214. [https://doi.org/10.1183/13993003.00214-2017]. - DOI - PubMed
-
- D'Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123–41. doi: 10.1186/s12931-014-0123-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

