Contacts between the endoplasmic reticulum and other membranes in neurons
- PMID: 28559323
- PMCID: PMC5474793
- DOI: 10.1073/pnas.1701078114
Contacts between the endoplasmic reticulum and other membranes in neurons
Abstract
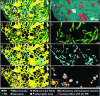
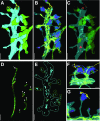
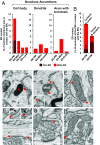
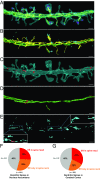
Close appositions between the membrane of the endoplasmic reticulum (ER) and other intracellular membranes have important functions in cell physiology. These include lipid homeostasis, regulation of Ca2+ dynamics, and control of organelle biogenesis and dynamics. Although these membrane contacts have previously been observed in neurons, their distribution and abundance have not been systematically analyzed. Here, we have used focused ion beam-scanning electron microscopy to generate 3D reconstructions of intracellular organelles and their membrane appositions involving the ER (distance ≤30 nm) in different neuronal compartments. ER-plasma membrane (PM) contacts were particularly abundant in cell bodies, with large, flat ER cisternae apposed to the PM, sometimes with a notably narrow lumen (thin ER). Smaller ER-PM contacts occurred throughout dendrites, axons, and in axon terminals. ER contacts with mitochondria were abundant in all compartments, with the ER often forming a network that embraced mitochondria. Small focal contacts were also observed with tubulovesicular structures, likely to be endosomes, and with sparse multivesicular bodies and lysosomes found in our reconstructions. Our study provides an anatomical reference for interpreting information about interorganelle communication in neurons emerging from functional and biochemical studies.
Keywords: FIB-SEM; Stim1; spine apparatus; subsurface cisternae; thin ER.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


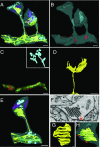
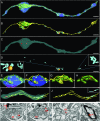
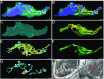
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

