Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana
- PMID: 28559333
- PMCID: PMC5474774
- DOI: 10.1073/pnas.1613499114
Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana
Abstract
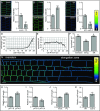
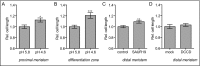
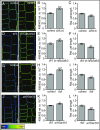
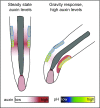
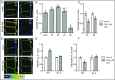
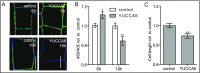
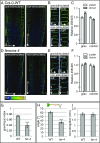
Plant cells are embedded within cell walls, which provide structural integrity, but also spatially constrain cells, and must therefore be modified to allow cellular expansion. The long-standing acid growth theory postulates that auxin triggers apoplast acidification, thereby activating cell wall-loosening enzymes that enable cell expansion in shoots. Interestingly, this model remains heavily debated in roots, because of both the complex role of auxin in plant development as well as technical limitations in investigating apoplastic pH at cellular resolution. Here, we introduce 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) as a suitable fluorescent pH indicator for assessing apoplastic pH, and thus acid growth, at a cellular resolution in Arabidopsis thaliana roots. Using HPTS, we demonstrate that cell wall acidification triggers cellular expansion, which is correlated with a preceding increase of auxin signaling. Reduction in auxin levels, perception, or signaling abolishes both the extracellular acidification and cellular expansion. These findings jointly suggest that endogenous auxin controls apoplastic acidification and the onset of cellular elongation in roots. In contrast, an endogenous or exogenous increase in auxin levels induces a transient alkalinization of the extracellular matrix, reducing cellular elongation. The receptor-like kinase FERONIA is required for this physiological process, which affects cellular root expansion during the gravitropic response. These findings pinpoint a complex, presumably concentration-dependent role for auxin in apoplastic pH regulation, steering the rate of root cell expansion and gravitropic response.
Keywords: apoplastic pH; auxin; cellular expansion; root gravitropism; root growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

