RNA-seq reveals conservation of function among the yolk sacs of human, mouse, and chicken
- PMID: 28559354
- PMCID: PMC5474779
- DOI: 10.1073/pnas.1702560114
RNA-seq reveals conservation of function among the yolk sacs of human, mouse, and chicken
Abstract
The yolk sac is phylogenetically the oldest of the extraembryonic membranes. The human embryo retains a yolk sac, which goes through primary and secondary phases of development, but its importance is controversial. Although it is known to synthesize proteins, its transport functions are widely considered vestigial. Here, we report RNA-sequencing (RNA-seq) data for the human and murine yolk sacs and compare those data with data for the chicken. We also relate the human RNA-seq data to proteomic data for the coelomic fluid bathing the yolk sac. Conservation of transcriptomes across the species indicates that the human secondary yolk sac likely performs key functions early in development, particularly uptake and processing of macro- and micronutrients, many of which are found in coelomic fluid. More generally, our findings shed light on evolutionary mechanisms that give rise to complex structures such as the placenta. We identify genetic modules that are conserved across mammals and birds, suggesting these modules are part of the core amniote genetic repertoire and are the building blocks for both oviparous and viviparous reproductive modes. We propose that although a choriovitelline placenta is never established physically in the human, the placental villi, the exocoelomic cavity, and the secondary yolk sac function together as a physiological equivalent.
Keywords: evolution; placenta; yolk sac.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
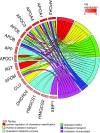
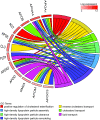




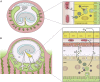
References
-
- Mossman H. Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology: Evolution; Phylogenetic Significance; Basic Functions, Research Opportunities. Macmillan; London: 1987.
-
- Brent RL, Fawcett LB. Nutritional studies of the embryo during early organogenesis with normal embryos and embryos exhibiting yolk sac dysfunction. J Pediatr. 1998;132:S6–S16. - PubMed
-
- Woollett LA. Where does fetal and embryonic cholesterol originate and what does it do? Annu Rev Nutr. 2008;28:97–114. - PubMed
-
- Baardman ME, et al. The origin of fetal sterols in second-trimester amniotic fluid: Endogenous synthesis or maternal-fetal transport? Am J Obstet Gynecol. 2012;207:202.e19–202.e25. - PubMed
-
- Baardman ME, et al. The role of maternal-fetal cholesterol transport in early fetal life: Current insights. Biol Reprod. 2013;88:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

