Circadian, Carbon, and Light Control of Expansion Growth and Leaf Movement
- PMID: 28559360
- PMCID: PMC5490918
- DOI: 10.1104/pp.17.00503
Circadian, Carbon, and Light Control of Expansion Growth and Leaf Movement
Abstract
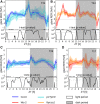
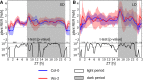
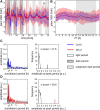
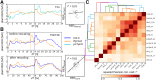
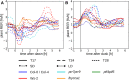
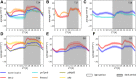
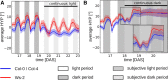
We used Phytotyping4D to investigate the contribution of clock and light signaling to the diurnal regulation of rosette expansion growth and leaf movement in Arabidopsis (Arabidopsis thaliana). Wild-type plants and clock mutants with a short (lhycca1) and long (prr7prr9) period were analyzed in a T24 cycle and in T-cycles that were closer to the mutants' period. Wild types also were analyzed in various photoperiods and after transfer to free-running light or darkness. Rosette expansion and leaf movement exhibited a circadian oscillation, with superimposed transients after dawn and dusk. Diurnal responses were modified in clock mutants. lhycca1 exhibited an inhibition of growth at the end of night and growth rose earlier after dawn, whereas prr7prr9 showed decreased growth for the first part of the light period. Some features were partly rescued by a matching T-cycle, like the inhibition in lhycca1 at the end of the night, indicating that it is due to premature exhaustion of starch. Other features were not rescued, revealing that the clock also regulates expansion growth more directly. Expansion growth was faster at night than in the daytime, whereas published work has shown that the synthesis of cellular components is faster in the day than at nighttime. This temporal uncoupling became larger in short photoperiods and may reflect the differing dependence of expansion and biosynthesis on energy, carbon, and water. While it has been proposed that leaf expansion and movement are causally linked, we did not observe a consistent temporal relationship between expansion and leaf movement.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Ache P, Bauer H, Kollist H, Al-Rasheid KA, Lautner S, Hartung W, Hedrich R (2010) Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J 62: 1072–1082 - PubMed
-
- Alabadí D, Yanovsky MJ, Más P, Harmer SL, Kay SA (2002) Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol 12: 757–761 - PubMed
-
- Apelt F, Breuer D, Nikoloski Z, Stitt M, Kragler F (2015) Phytotyping4D: a light-field imaging system for non-invasive and accurate monitoring of spatio-temporal plant growth. Plant J 82: 693–706 - PubMed
-
- Boardman N. (1977) Comparative photosynthesis of sun and shade plants. Annu Rev Plant Physiol 28: 355–377
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

