Bitter taste receptors as targets for tocolytics in preterm labor therapy
- PMID: 28559440
- PMCID: PMC5572693
- DOI: 10.1096/fj.201601323RR
Bitter taste receptors as targets for tocolytics in preterm labor therapy
Abstract
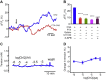
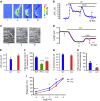
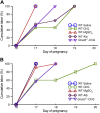
Preterm birth (PTB) is the leading cause of neonatal mortality and morbidity, with few prevention and treatment options. Uterine contraction is a central feature of PTB, so gaining new insights into the mechanisms of this contraction and consequently identifying novel targets for tocolytics are essential for more successful management of PTB. Here we report that myometrial cells from human and mouse express bitter taste receptors (TAS2Rs) and their canonical signaling components (i.e., G-protein gustducin and phospholipase C β2). Bitter tastants can completely relax myometrium precontracted by different uterotonics. In isolated single mouse myometrial cells, a phenotypical bitter tastant (chloroquine, ChQ) reverses the rise in intracellular Ca2+ concentration ([Ca2+]i) and cell shortening induced by uterotonics, and this reversal effect is inhibited by pertussis toxin and by genetic deletion of α-gustducin. In human myometrial cells, knockdown of TAS2R14 but not TAS2R10 inhibits ChQ's reversal effect on an oxytocin-induced rise in [Ca2+]i Finally, ChQ prevents mouse PTBs induced by bacterial endotoxin LPS or progesterone receptor antagonist mifepristone more often than current commonly used tocolytics, and this prevention is largely lost in α-gustducin-knockout mice. Collectively, our results reveal that activation of the canonical TAS2R signaling system in myometrial cells produces profound relaxation of myometrium precontracted by a broad spectrum of contractile agonists, and that targeting TAS2Rs is an attractive approach to developing effective tocolytics for PTB management.-Zheng, K., Lu, P., Delpapa, E., Bellve, K., Deng, R., Condon, J. C., Fogarty, K., Lifshitz, L. M., Simas, T. A. M., Shi, F., ZhuGe, R. Bitter taste receptors as targets for tocolytics in preterm labor therapy.
Keywords: G-protein coupled receptor; chloroquine; relaxation; uterine smooth muscle.
© FASEB.
Figures
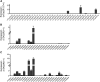
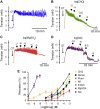
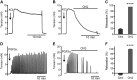
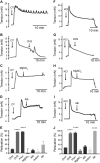

References
-
- Erdei C., Dammann O. (2014) The perfect storm: preterm birth, neurodevelopmental mechanisms, and autism causation. Perspect. Biol. Med. 57, 470–481 - PubMed
-
- Ortinau C., Neil J. (2015) The neuroanatomy of prematurity: normal brain development and the impact of preterm birth. Clin. Anat. 28, 168–183 - PubMed
-
- Simmons L. E., Rubens C. E., Darmstadt G. L., Gravett M. G. (2010) Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin. Perinatol. 34, 408–415 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

