Magnetic Resonance Imaging of Cardiovascular Function and the Brain: Is Dementia a Cardiovascular-Driven Disease?
- PMID: 28559496
- PMCID: PMC5475278
- DOI: 10.1161/CIRCULATIONAHA.116.021978
Magnetic Resonance Imaging of Cardiovascular Function and the Brain: Is Dementia a Cardiovascular-Driven Disease?
Abstract
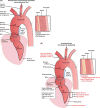
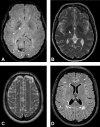
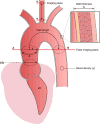
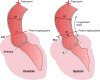
The proximal aorta acts as a coupling device between heart and brain perfusion, modulating the amount of pressure and flow pulsatility transmitted into the cerebral microcirculation. Stiffening of the proximal aorta is strongly associated with age and hypertension. The detrimental effects of aortic stiffening may result in brain damage as well as heart failure. The resulting cerebral small vessel disease and heart failure may contribute to early cognitive decline and (vascular) dementia. This pathophysiological sequence of events underscores the role of cardiovascular disease as a contributory mechanism in causing cognitive decline and dementia and potentially may provide a starting point for prevention and treatment. Magnetic resonance imaging is well suited to assess the function of the proximal aorta and the left ventricle (eg, aortic arch pulse wave velocity and distensibility) as well as the various early and late manifestations of cerebral small vessel disease (eg, microbleeds and white matter hyperintensities in strategically important regions of the brain). Specialized magnetic resonance imaging techniques are explored for diagnosing preclinical changes in white matter integrity or brain microvascular pulsatility.
Keywords: aorta; brain; cardiovascular disease; magnetic resonance imaging; pulse wave velocity.
© 2017 American Heart Association, Inc.
Figures

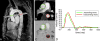
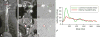
References
-
- Jones DS, Greene JA. Is Dementia in Decline? Historical Trends and Future Trajectories. The New England journal of medicine. 2016;374:507–509. - PubMed
-
- Angermann CE, Frey A, Ertl G. Cognition matters in cardiovascular disease and heart failure. European heart journal. 2012;33:1721–1723. - PubMed
-
- Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP, Investigators H-A Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. Jama. 2009;301:1451–1459. - PMC - PubMed
-
- Deshields TL, McDonough EM, Mannen RK, Miller LW. Psychological and cognitive status before and after heart transplantation. General hospital psychiatry. 1996;18:62S–69S. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

