Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization
- PMID: 28559722
- PMCID: PMC5436388
- DOI: 10.1016/j.sjopt.2017.02.008
Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization
Abstract
In order to evaluate the effect of topical and subconjunctival anti-vascular endothelial growth factor (anti-VEGF) therapy, Ranibizumab, Bevacizumab and Aflibercept as a therapy for corneal neovascularization (NV) treatment, the aim of this study was to review all data related to some of anti-VEGF as a promising therapies for corneal NV treatment. Corneal NV is a dangerous condition leading to a marked reduction in vision due to angiogenesis of abnormal vessels that block light. During the recent years, we have recognized new drug proliferation for corneal NV treatment. Recently, anti-VEGF therapies are one of the most important drugs used for corneal NV treatment. Several growth factors are involved in angiogenesis. The most important growth factor in corneal angiogenesis is VEGF. VEGF can be considered as key mediators in corneal angiogenesis. It is upregulated during corneal NV. In fact, anti-VEGF therapies have shown efficacy in attenuation of corneal NV in both animal models and clinical trials. A promising therapeutic success has been achieved using antibodies directed against VEGF. Bevacizumab has demonstrated efficacy and efficiency in the treatment of different neo-vascular ocular diseases and it has partially reduced corneal NV through different routes of administrations: topical, subconjunctival, and intraocular application. A similar efficacy to bevacizumab profiles in the treatment of neo-vascular age-related macular degeneration was induced by ranibizumab. Moreover, at worse levels of initial visual acuity of diabetic macular edema, aflibercept was more effective at improving vision. Anti-VEGF agents (Bevacizumab, Ranibizumab and Aflibercept) seem to have a higher efficiency and efficacy for corneal NV treatment. Both subconjunctival therapy and topical therapy of bevacizumab prohibit corneal NV, while early treatment with subconjunctival administration of ranibizumab may successfully reduce corneal NV. Therefore, establishment of safe doses is highly important before these drugs can be involved in the clinical setting. Further investigations and studies are highly warranted to adjust the dose and route of administration for the antibodies directed against VEGF to be the key therapeutic agents in the corneal NV treatment.
Keywords: Aflibercept; Anti-VEGF; Bevacizumab; Cornea; Ranibizumab.
Figures
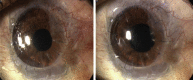
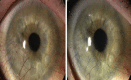
References
-
- DelMonte D.W., Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. - PubMed
-
- Streilein J.W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. - PubMed
-
- Wagoner M.D. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275–313. - PubMed
-
- Brodovsky S.C., McCarty C.A., Snibson G. Management of alkali burns: an 11 year retrospective review. Ophthalmology. 2000;107:1829–1835. - PubMed
-
- Saika S., Kobata S., Hashizume N. Epithelial basement membrane in alkali-burned corneas in rats. Immunohistochemical study. Cornea. 1993;12:383–390. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

