Oncolytic Herpes Simplex Viral Therapy: A Stride toward Selective Targeting of Cancer Cells
- PMID: 28559846
- PMCID: PMC5432606
- DOI: 10.3389/fphar.2017.00270
Oncolytic Herpes Simplex Viral Therapy: A Stride toward Selective Targeting of Cancer Cells
Abstract
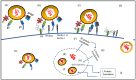
Oncolytic viral therapy, which makes use of replication-competent lytic viruses, has emerged as a promising modality to treat malignancies. It has shown meaningful outcomes in both solid tumor and hematologic malignancies. Advancements during the last decade, mainly genetic engineering of oncolytic viruses have resulted in improved specificity and efficacy of oncolytic viruses in cancer therapeutics. Oncolytic viral therapy for treating cancer with herpes simplex virus-1 has been of particular interest owing to its range of benefits like: (a) large genome and power to infiltrate in the tumor, (b) easy access to manipulation with the flexibility to insert multiple transgenes, (c) infecting majority of the malignant cell types with quick replication in the infected cells and (d) as Anti-HSV agent to terminate HSV replication. This review provides an exhaustive list of oncolytic herpes simplex virus-1 along with their genetic alterations. It also encompasses the major developments in oncolytic herpes simplex-1 viral therapy and outlines the limitations and drawbacks of oncolytic herpes simplex viral therapy.
Keywords: cancer; genetic arming; herpes simplex virus; oncolytic viral therapy; retargeted oncolytic herpes simplex virus-1.
Figures
References
-
- Anderson D. B., Laquerre S., Ghosh K., Ghosh H. P., Goins W. F., Cohen J. B., et al. (2000). Pseudotyping of glycoprotein D-deficient herpes simplex virus type 1 with vesicular stomatitis virus glycoprotein G enables mutant virus attachment and entry. J. Virol. 74, 2481–2487. 10.1128/JVI.74.5.2481-2487.2000 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

