Genome-wide characterization of cellulases from the hemi-biotrophic plant pathogen, Bipolaris sorokiniana, reveals the presence of a highly stable GH7 endoglucanase
- PMID: 28559926
- PMCID: PMC5445349
- DOI: 10.1186/s13068-017-0822-0
Genome-wide characterization of cellulases from the hemi-biotrophic plant pathogen, Bipolaris sorokiniana, reveals the presence of a highly stable GH7 endoglucanase
Abstract
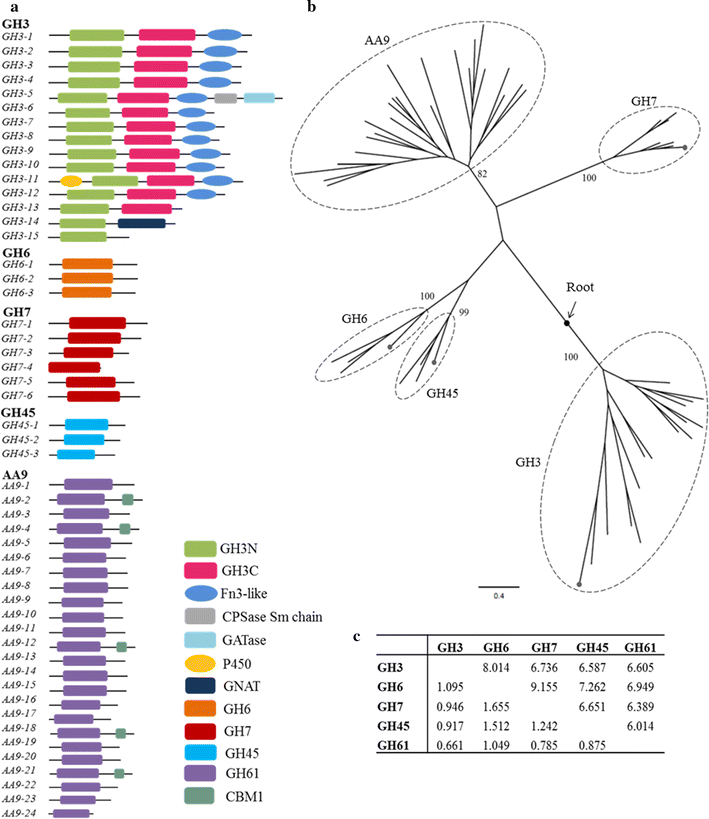
Background: Bipolaris sorokiniana is a filamentous fungus that causes spot blotch disease in cereals like wheat and has severe economic consequences. However, information on the identities and role of the cell wall-degrading enzymes (CWDE) in B. sorokiniana is very limited. Several fungi produce CWDE like glycosyl hydrolases (GHs) that help in host cell invasion. To understand the role of these CWDE in B. sorokiniana, the first step is to identify and annotate all possible genes of the GH families like GH3, GH6, GH7, GH45 and AA9 and then characterize them biochemically.
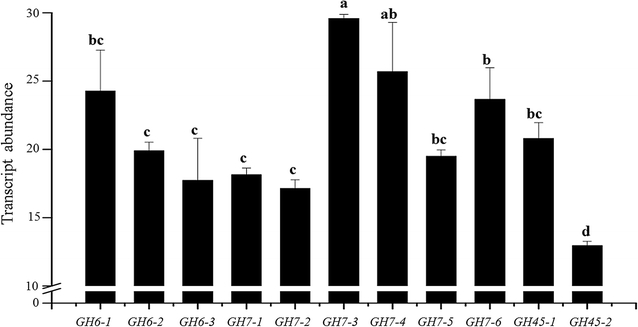
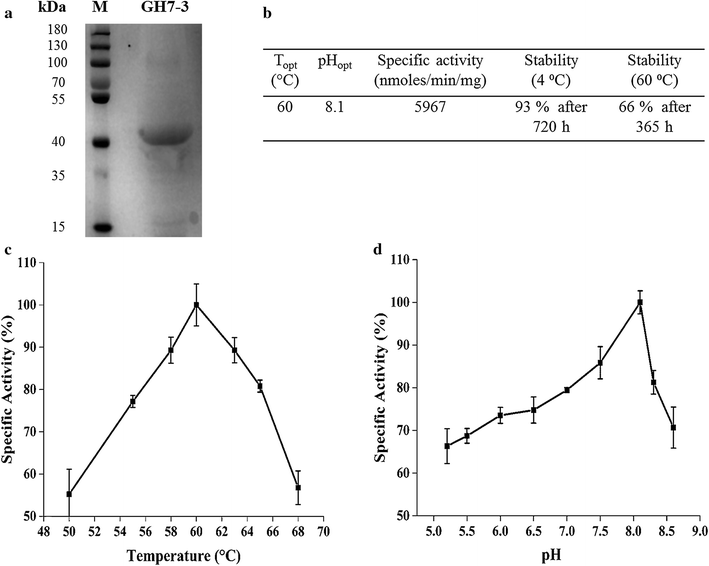
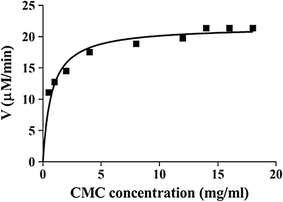
Results: We confirmed and annotated the homologs of GH3, GH6, GH7, GH45 and AA9 enzymes in the B. sorokiniana genome using the sequence and domain features of these families. Quantitative real-time PCR analyses of these homologs revealed that the transcripts of the BsGH7-3 (3rd homolog of the GH 7 family in B. sorokiniana) were most abundant. BsGH7-3, the gene of BsGH7-3, was thus cloned into pPICZαC Pichia pastoris vector and expressed in X33 P. pastoris host to be characterized. BsGH7-3 enzyme showed a temperature optimum of 60 °C and a pHopt of 8.1. BsGH7-3 was identified to be an endoglucanase based on its broad substrate specificity and structural comparisons with other such endoglucanases. BsGH7-3 has a very long half-life and retains 100% activity even in the presence of 4 M NaCl, 4 M KCl and 20% (v/v) ionic liquids. The enzyme activity is stimulated up to fivefold in the presence of Mn+2 and Fe+2 without any deleterious effects on enzyme thermostability.
Conclusions: Here we reanalysed the B. sorokiniana genome and selected one GH7 enzyme for further characterization. The present work demonstrates that BsGH7-3 is an endoglucanase with a long half-life and no loss in activity in the presence of denaturants like salt and ionic liquids, and lays the foundation towards exploring the Bipolaris genome for other cell wall-degrading enzymes.
Keywords: Alkaliphilic; Bipolaris sorokiniana; Cell wall-degrading enzymes; GH7 endoglucanases; Glycosyl hydrolase; Ionic liquids; Salt tolerant; Thermostable.
Figures

References
-
- Durand H, Clanet M, Tiraby G. Genetic improvement of Trichoderma reesei for large scale cellulase production. Enzyme Microbiol Technol. 1988;10(6):341–346. doi: 10.1016/0141-0229(88)90012-9. - DOI
-
- Harris PV, Welner D, McFarland KC, Re E, Navarro Poulsen JC, Brown K, Salbo R, Ding H, Vlasenko E, Merino S, et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry. 2010;49(15):3305–3316. doi: 10.1021/bi100009p. - DOI - PubMed
-
- Datta S. Recent strategies to overexpress and engineer cellulases for biomass degradation. Curr Metabol. 2016;4(1):14–22. doi: 10.2174/2213235X03666150702155845. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

