Application and evaluation of NODDI in the cervical spinal cord of multiple sclerosis patients
- PMID: 28560158
- PMCID: PMC5443965
- DOI: 10.1016/j.nicl.2017.05.010
Application and evaluation of NODDI in the cervical spinal cord of multiple sclerosis patients
Abstract
Introduction: There is a need to develop imaging methods sensitive to axonal injury in multiple sclerosis (MS), given the prominent impact of axonal pathology on disability and outcome. Advanced multi-compartmental diffusion models offer novel indices sensitive to white matter microstructure. One such model, neurite orientation dispersion and density imaging (NODDI), is sensitive to neurite morphology, providing indices of apparent volume fractions of axons (vin), isotropic water (viso) and the dispersion of fibers about a central axis (orientation dispersion index, ODI). NODDI has yet to be studied for its sensitivity to spinal cord pathology. Here, we investigate the feasibility and utility of NODDI in the cervical spinal cord of MS patients.
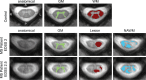
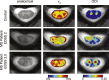
Methods: NODDI was applied in the cervical spinal cord in a cohort of 8 controls and 6 MS patients. Statistical analyses were performed to test the sensitivity of NODDI-derived indices to pathology in MS (both lesion and normal appearing white matter NAWM). Diffusion kurtosis imaging (DKI) and diffusion tensor imaging (DTI) analysis were also performed to compare with NODDI.
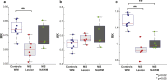
Results: A decrease in NODDI-derived vin was observed at the site of the lesion (p < 0.01), whereas a global increase in ODI was seen throughout white matter (p < 0.001). DKI-derived mean kurtosis (MK) and radial kurtosis (RK) and DTI-derived fractional anisotropy (FA) and radial diffusivity (RD) were all significantly different in MS patients (p < 0.02), however NODDI provided higher contrast between NAWM and lesion in all MS patients.
Conclusion: NODDI provides unique contrast that is not available with DKI or DTI, enabling improved characterization of the spinal cord in MS.
Keywords: Axon; Diffusion; Multiple sclerosis; NODDI; Spinal cord; Volume fraction.
Figures






References
-
- Alexander D.C. A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magn. Reson. Med. 2008;60:439–448. - PubMed
-
- Assaf Y., Basser P.J. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. NeuroImage. 2005;27:48–58. - PubMed
-
- Assaf Y., Cohen Y. Assignment of the water slow-diffusing component in the central nervous system using q-space diffusion MRS: implications for fiber tract imaging. Magn. Reson. Med. 2000;43:191–199. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

