Data demonstrating the anti-oxidant role of hemopexin in the heart
- PMID: 28560284
- PMCID: PMC5443894
- DOI: 10.1016/j.dib.2017.05.026
Data demonstrating the anti-oxidant role of hemopexin in the heart
Abstract
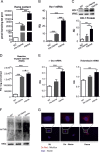
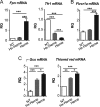
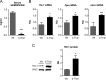
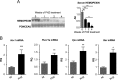
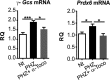
The data presented in this article are related to the research article entitled Hemopexin counteracts systolic dysfunction induced by heme-driven oxidative stress (G. Ingoglia, C. M. Sag, N. Rex, L. De Franceschi, F. Vinchi, J. Cimino, S. Petrillo, S. Wagner, K. Kreitmeier, L. Silengo, F. Altruda, L. S. Maier, E. Hirsch, A. Ghigo and E. Tolosano, 2017) [1]. Data show that heme induces reactive oxygen species (ROS) production in primary cardiomyocytes. H9c2 myoblastic cells treated with heme bound to human Hemopexin (Hx) are protected from heme accumulation and oxidative stress. Similarly, the heme-driven oxidative response is reduced in primary cardiomyocytes treated with Hx-heme compared to heme alone. Our in vivo data show that mouse models of hemolytic disorders, β-thalassemic mice and phenylhydrazine-treated mice, have low serum Hx associated to enhanced expression of heme- and oxidative stress responsive genes in the heart. Hx-/- mice do not show signs of heart fibrosis or overt inflammation. For interpretation and discussion of these data, refer to the research article referenced above.
Keywords: Heart; Heme; Hemopexin; Oxidative stress.
Figures

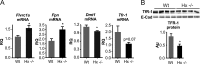

References
-
- Ingoglia G., Sag C.M., Rex N., De Franceschi L., Vinchi F., Cimino J., Petrillo S., Wagner S., Kreitmeier K., Silengo L., Altruda F., Maier L.S., Hirsch E., Ghigo A., Tolosano E. Hemopexin counteracts systolic dysfunction induced by heme-driven oxidative stress. Free Radic Biol Med. 2017;108:452–464. - PubMed
-
- Tolosano E., Hirsch E., Patrucco E., Camaschella C., Navone R., Silengo L., Altruda F. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94(11):3906–3914. - PubMed
-
- Franco S.S., De Falco L., Ghaffari S., Brugnara C., Sinclair D.A., Matte׳ A., Iolascon A., Mohandas N., Bertoldi M., An X., Siciliano A., Rimmelé P., Cappellini M.D., Michan S., Zoratti E., Anne J., Franceschi L. De. Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2014;99(2):267–275. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

