Cyclical nursing patterns in wild orangutans
- PMID: 28560319
- PMCID: PMC5435413
- DOI: 10.1126/sciadv.1601517
Cyclical nursing patterns in wild orangutans
Abstract
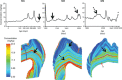
Nursing behavior is notoriously difficult to study in arboreal primates, particularly when offspring suckle inconspicuously in nests. Orangutans have the most prolonged nursing period of any mammal, with the cessation of suckling (weaning) estimated to occur at 6 to 8 years of age in the wild. Milk consumption is hypothesized to be relatively constant over this period, but direct evidence is limited. We previously demonstrated that trace element analysis of bioavailable elements from milk, such as barium, provides accurate estimates of early-life diet transitions and developmental stress when coupled with growth lines in the teeth of humans and nonhuman primates. We provide the first detailed nursing histories of wild, unprovisioned orangutans (Pongo abelii and Pongo pygmaeus) using chemical and histological analyses. Laser ablation inductively coupled plasma mass spectrometry was used to determine barium distributions across the teeth of four wild-shot individuals aged from postnatal biological rhythms. Barium levels rose during the first year of life in all individuals and began to decline shortly after, consistent with behavioral observations of intensive nursing followed by solid food supplementation. Subsequent barium levels show large sustained fluctuations on an approximately annual basis. These patterns appear to be due to cycles of varying milk consumption, continuing until death in an 8.8-year-old Sumatran individual. A female Bornean orangutan ceased suckling at 8.1 years of age. These individuals exceed the maximum weaning age reported for any nonhuman primate. Orangutan nursing may reflect cycles of infant demand that relate to fluctuating resource availability.
Keywords: Seasonality; barium; life history; non-human primates; nursing; orangutan; weaning.
Figures




Similar articles
-
Oxygen isotopes in orangutan teeth reveal recent and ancient climate variation.Elife. 2024 Mar 8;12:RP90217. doi: 10.7554/eLife.90217. Elife. 2024. PMID: 38457350 Free PMC article.
-
The slow ape: High infant survival and long interbirth intervals in wild orangutans.J Hum Evol. 2018 Dec;125:38-49. doi: 10.1016/j.jhevol.2018.09.004. Epub 2018 Oct 25. J Hum Evol. 2018. PMID: 30502896
-
Feeding behavior, diet, and the functional consequences of jaw form in orangutans, with implications for the evolution of Pongo.J Hum Evol. 2006 Apr;50(4):377-93. doi: 10.1016/j.jhevol.2005.10.006. Epub 2006 Jan 18. J Hum Evol. 2006. PMID: 16413045
-
Slow loris (Nycticebus borneanus) consumption by a wild Bornean orangutan (Pongo pygmaeus wurmbii).Primates. 2022 Jan;63(1):25-31. doi: 10.1007/s10329-021-00960-4. Primates. 2022. PMID: 34787739
-
Not by science alone: why orangutan conservationists must think outside the box.Ann N Y Acad Sci. 2012 Feb;1249:29-44. doi: 10.1111/j.1749-6632.2011.06288.x. Epub 2011 Dec 16. Ann N Y Acad Sci. 2012. PMID: 22175247 Review.
Cited by
-
Effects of Different Generations and Sex on Physiological, Biochemical, and Growth Parameters of Crossbred Beef Cattle by Myostatin Gene-Edited Luxi Bulls and Simmental Cows.Animals (Basel). 2023 Oct 14;13(20):3216. doi: 10.3390/ani13203216. Animals (Basel). 2023. PMID: 37893940 Free PMC article.
-
Synchrotron X-ray Fluorescence Microscopy Reveals Trace Elemental Indicators of Life History in Marsupial Teeth.Biol Trace Elem Res. 2025 Sep;203(9):4607-4619. doi: 10.1007/s12011-024-04502-z. Epub 2025 Jan 16. Biol Trace Elem Res. 2025. PMID: 39821184 Free PMC article.
-
Wintertime stress, nursing, and lead exposure in Neanderthal children.Sci Adv. 2018 Oct 31;4(10):eaau9483. doi: 10.1126/sciadv.aau9483. eCollection 2018 Oct. Sci Adv. 2018. PMID: 30402544 Free PMC article.
-
Oxygen isotopes in orangutan teeth reveal recent and ancient climate variation.Elife. 2024 Mar 8;12:RP90217. doi: 10.7554/eLife.90217. Elife. 2024. PMID: 38457350 Free PMC article.
-
Ancient DNA reveals monozygotic newborn twins from the Upper Palaeolithic.Commun Biol. 2020 Nov 6;3(1):650. doi: 10.1038/s42003-020-01372-8. Commun Biol. 2020. PMID: 33159107 Free PMC article.
References
-
- Lee P. C., Majluf P., Gordon I. J., Growth, weaning and maternal investment from a comparative perspective. J. Zool. 225, 99–114 (1991).
-
- Pontzer H., Brown M. H., Raichlen D. A., Dunsworth H., Hare B., Walker K., Luke A., Dugas L. R., Durazo-Arvizu R., Schoeller D., Plange-Rhule J., Bovet P., Forrester T. E., Lambert E. V., Thompson M. E., Shumaker R. W., Ross S. R., Metabolic acceleration and the evolution of human brain size and life history. Nature 533, 390–392 (2016). - PMC - PubMed
-
- Wich S. A., Utami-Atmoko S. S., Setia T. M., Rijksen H. D., Schürmann C., van Hooff J. A. R. A. M., van Schaik C. P., Life history of wild Sumatran orangutans (Pongo abelii). J. Hum. Evol. 47, 385–398 (2004). - PubMed
-
- C. D. Knott, M. E. Thompson, S. A. Wich, in Orangutans: Geographic Variation in Behavioral Ecology and Conservation, S. A. Wich, S. S. Utami Atmoko, T. M. Setia, C. P. van Schaik, Eds. (Oxford Univ. Press, 2009), pp. 171–188.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

