The PI3K/Akt/mTOR pathway is involved in CVB3-induced autophagy of HeLa cells
- PMID: 28560385
- PMCID: PMC5466389
- DOI: 10.3892/ijmm.2017.3008
The PI3K/Akt/mTOR pathway is involved in CVB3-induced autophagy of HeLa cells
Abstract
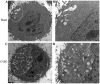
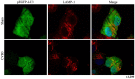
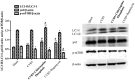
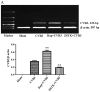
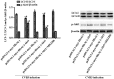
Recent studies have found that viral myocarditis (VMC) associated with coxsackievirus B3 (CVB3) causes autophagy activation after infection, but the specific mechanism is not clear. The present study demonstrated that the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB)/mammalian target of rapamycin (mTOR) signaling pathway participates in CVB3‑induced autophagy. We found that the light chain 3 (LC3)‑Ⅱ/LC3‑I ratio was increased and p62 and p‑mTOR were altered at different times during CVB3 infection. To further assess the effects of this signaling pathway on CVB3 infection and viral replication, we selected 24 h post‑inoculation (h.p.i.) as our research time point to conduct our next study. We inhibited the function of PI3K, Akt1 and mTOR. The outcome showed that inhibition of PI3K with ZSTK474 alleviated autophagy and decreased CVB3 mRNA replication and VP1 expression. Inhibition of mTOR with rapamycin promoted autophagy and viral mRNA replication but did not impact VP1 expression. Inhibition of Akt with MK2206 aggravated autophagy induced by viral infection. In our research, p62 exhibited a decrease at the beginning of infection but then increased as infection time increased. This finding may serve as a clue to elucidate the function of autophagy at different times of infection. However, the details merit further study. In conclusion, our findings suggest that the PI3K/Akt/mTOR signaling pathway participates in the process of autophagy induced by CVB3 infection. This finding may provide a new perspective of CVB3-induced autophagy.
Figures








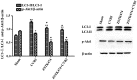

References
-
- Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/S0735-1097(03)00648-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

