Collagen-derived N-acetylated proline-glycine-proline upregulates the expression of pro-inflammatory cytokines and extracellular matrix proteases in nucleus pulposus cells via the NF-κB and MAPK signaling pathways
- PMID: 28560408
- PMCID: PMC5466390
- DOI: 10.3892/ijmm.2017.3005
Collagen-derived N-acetylated proline-glycine-proline upregulates the expression of pro-inflammatory cytokines and extracellular matrix proteases in nucleus pulposus cells via the NF-κB and MAPK signaling pathways
Abstract
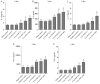
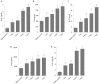
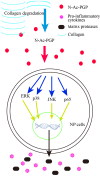
N-acetylated proline-glycine-proline (N-Ac-PGP) is a chemokine involved in inflammatory diseases and is found to accumulate in degenerative discs. N-Ac-PGP has been demonstrated to have a pro-inflammatory effect on human cartilage endplate stem cells. However, the effect of N-Ac-PGP on human intervertebral disc cells, especially nucleus pulposus (NP) cells, remains unknown. The purpose of this study was to investigate the effect of N-Ac-PGP on the expression of pro-inflammatory factors and extracellular matrix (ECM) proteases in NP cells and the molecular mechanism underlying this effect. Therefore, Milliplex assays were used to detect the levels of various inflammatory cytokines in conditioned culture medium of NP cells treated with N-Ac-PGP, including interleukin-1β (IL-1β), IL-6, IL-17, tumor necrosis factor-α (TNF-α) and C-C motif ligand 2 (CCL2). RT-qPCR was also used to determine the expression of pro-inflammatory cytokines and ECM proteases in the NP cells treated with N-Ac-PGP. Moreover, the role of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways in mediating the effect of N-Ac-PGP on the phenotype of NP cells was investigated using specific signaling inhibitors. Milliplex assays showed that NP cells treated with N-Ac-PGP (10 and 100 µg/ml) secreted higher levels of IL-1β, IL-6, IL-17, TNF-α and CCL2 compared with the control. RT-qPCR assays showed that NP cells treated with N-Ac-PGP (100 µg/ml) had markedly upregulated expression of matrix metalloproteinase 3 (MMP3), MMP13, a disintegrin and metalloproteinase with thrombospondin motif 4 (ADAMTS4), ADAMTS5, IL-6, CCL-2, CCL-5 and C-X-C motif chemokine ligand 10 (CXCL10). Moreover, N-Ac-PGP was shown to activate the MAPK and NF-κB signaling pathways in NP cells. MAPK and NF-κB signaling inhibitors suppressed the upregulation of proteases and pro-inflammatory cytokines in NP cells treated with N-Ac-PGP. In conclusion, N-Ac-PGP induces the expression of pro-inflammatory cytokines and matrix catabolic enzymes in NP cells via the NF-κB and MAPK signaling pathways. N-Ac-PGP is a novel therapeutic target for intervertebral disc degeneration.
Figures






References
-
- Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

