Impact of the Charge Ratio on the In Vivo Immunogenicity of Lipoplexes
- PMID: 28560696
- PMCID: PMC10601992
- DOI: 10.1007/s11095-017-2187-2
Impact of the Charge Ratio on the In Vivo Immunogenicity of Lipoplexes
Abstract
Purpose: The present study investigated the immunogenic potential of different cationic liposome formulations with a DNA plasmid encoding Pfs25, a malaria transmission-blocking vaccine candidate.
Methods: Pfs25 plasmid DNA was complexed with cationic liposomes to produce lipoplexes at different charge ratios of the cationic lipid head group to the nucleotide phosphate (N:P). The formation of lipoplexes was visualized by Cryogenic-TEM. Confocal microscopy of lipoplexes formed with GFP encoding plasmid DNA, and flow cytometry was used to determine their in vitro transfection capability. Two different lipoplex formulations using plasmid DNA encoding Pfs25 were evaluated for in vivo immunogenicity after intramuscular administration in Balb/c mice. Immune sera were analyzed by ELISA.
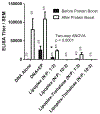
Results: The results demonstrated that the cationic liposome-mediated DNA immunization with an N:P charge ratio of 1:3 (anionic lipoplexes) is more effective than the use of naked plasmid DNA alone. No antibody response was observed when lipoplexes with a higher N:P charge ratio of 10:3 (cationic lipoplexes) were used. Trehalose was added to some lipoplex formulations as a cryoprotectant and adjuvant, but it did not yield any further improvement of immunogenicity in vivo.
Conclusions: The results suggest that Pfs25 plasmid DNA delivered as lipoplexes at a charge ratio of 1:3 elicited strong immunogenicity in mice and may be improved further to match the immune responses of DNA vaccines administered by in vivo electroporation.
Keywords: Pfs25 DNA; cationic liposome; lipoplex; malaria; vaccine.
Conflict of interest statement
Figures




References
-
- Kaslow DC, Bathurst IC, Barr PJ. Malaria transmission-blocking vaccines. Trends Biotechnol. 1992;10:388–91. - PubMed
-
- Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V. Chapter Three - Development of Malaria Transmission-Blocking Vaccines: From Concept to Product. In: Rollinson D, Stothard JR, editors. Advances in Parasitology. San Diego: Academic Press; 2015. p. 109–52. - PubMed
-
- Kaslow DC, Quakyi IA, Keister DB. Minimal variation in a vaccine candidate from the sexual stage of Plasmodium falciparum. Mol Biochem Parasitol. 1989;32(1):101–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

