Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination
- PMID: 28560740
- PMCID: PMC5518437
- DOI: 10.1002/glia.23167
Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination
Abstract
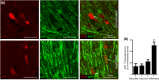
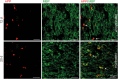
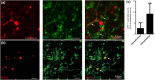
Remyelination is in the center of new therapies for the treatment of multiple sclerosis to resolve and improve disease symptoms and protect axons from further damage. Although remyelination is considered beneficial in the long term, it is not known, whether this is also the case early in lesion formation. Additionally, the precise timing of acute axonal damage and remyelination has not been assessed so far. To shed light onto the interrelation between axons and the myelin sheath during de- and remyelination, we employed cuprizone- and focal lysolecithin-induced demyelination and performed time course experiments assessing the evolution of early and late stage remyelination and axonal damage. We observed damaged axons with signs of remyelination after cuprizone diet cessation and lysolecithin injection. Similar observations were made in early multiple sclerosis lesions. To assess the correlation of remyelination and axonal damage in multiple sclerosis lesions, we took advantage of a cohort of patients with early and late stage remyelinated lesions and assessed the number of APP- and SMI32- positive damaged axons and the density of SMI31-positive and silver impregnated preserved axons. Early de- and remyelinating lesions did not differ with respect to axonal density and axonal damage, but we observed a lower axonal density in late stage demyelinated multiple sclerosis lesions than in remyelinated multiple sclerosis lesions. Our findings suggest that remyelination may not only be protective over a long period of time, but may play an important role in the immediate axonal recuperation after a demyelinating insult.
Keywords: axonal damage; multiple sclerosis; remyelination.
© 2017 The Authors GLIA Published by Wiley Periodicals, Inc.
Figures




References
-
- Bramlett, HM. , Kraydieh, S. , Green, E. J. , & Dietrich, W. D. (1997). Temporal and regional patterns of axonal damage following traumatic brain injury: a beta‐amyloid precursor protein immunocytochemical study in rats. Journal of Neuropathology & Experimental Neurology 56:1132–1141. - PubMed
-
- Brück, W. , Porada, P. , Poser, S. , Rieckmann, P. , Hanefeld, F. , Kretzschmar, HA. , & Lassmann, H . (1995). Monocyte/macrophage differentiation in early multiple sclerosis lesions. Annals of Neurology 38:788–796. - PubMed
-
- Brück, W. , Schmied, M. , Suchanek, G. , Brück, Y. , Breitschopf, H , Poser, S. , … Lassmann, H. (1994). Oligodendrocytes in the early course of multiple sclerosis. Annals of Neurology 35:65–73. - PubMed
-
- Clark, K. C. , Josephson, A. , Benusa, S. D. , Hartley, R. K. , Baer, M. , Thummala, S. , … Dupree, J. L. (2016). Compromised axon initial segment integrity in EAE is preceded by microglial reactivity and contact. Glia 64:1190–1209. - PubMed
-
- Cunningham, C. (2013). Microglia and neurodegeneration: the role of systemic inflammation. Glia 61:71–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

